The Exergy Loss Distribution and the Heat Transfer Capability in Subcritical Organic Rankine Cycle
Abstract
:1. Introduction
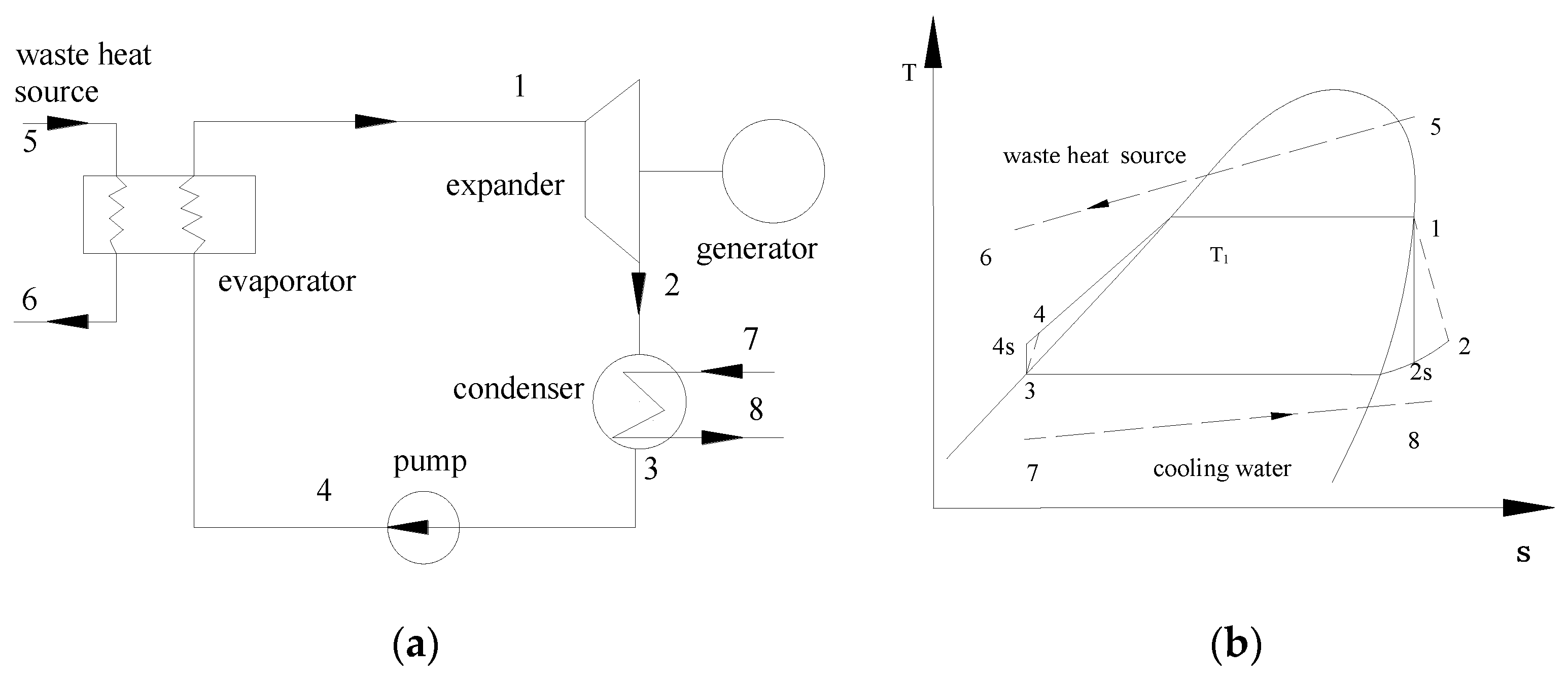
2. System Description and Assumptions
3. Mathematical Model
4. Results and Analysis
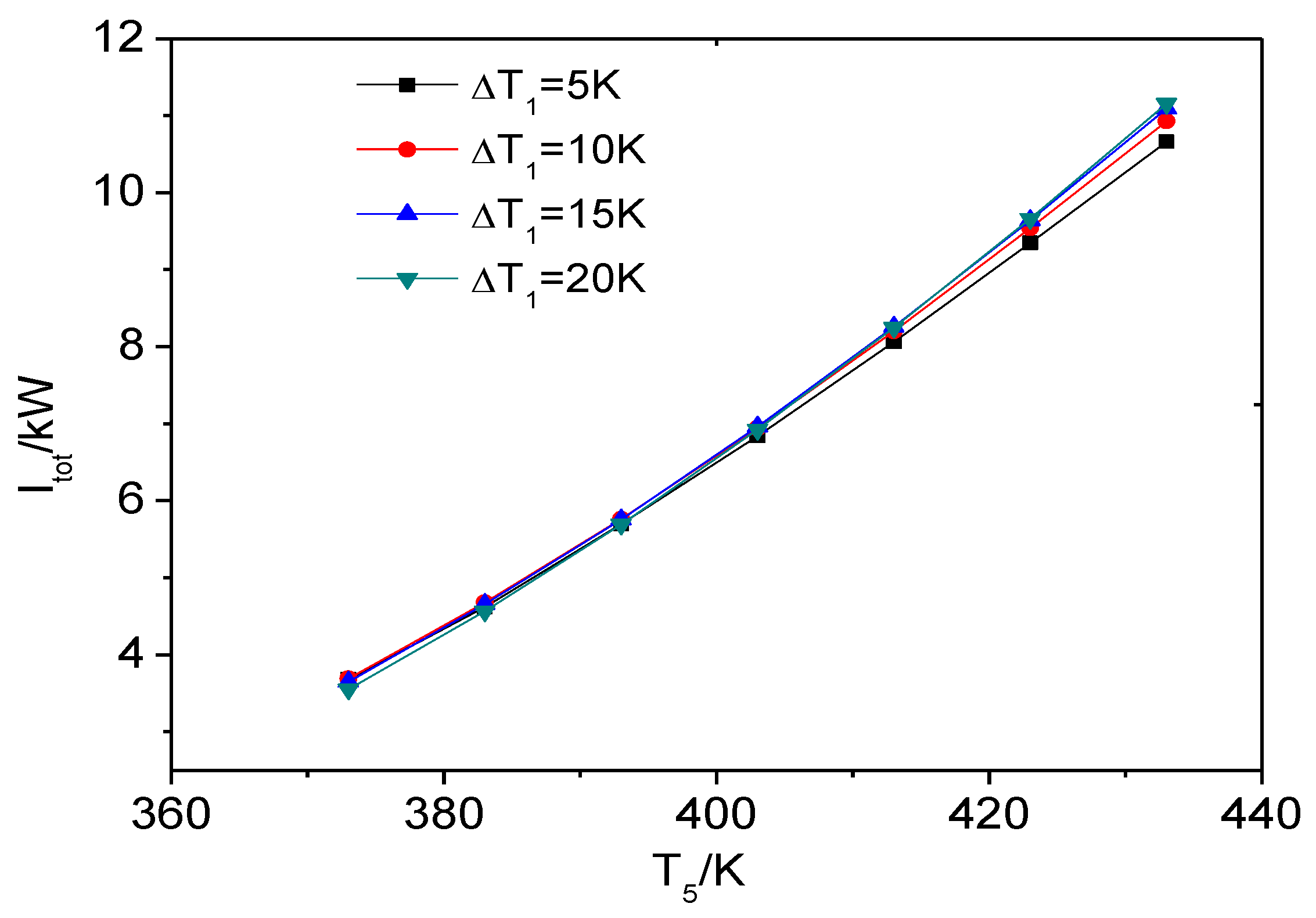
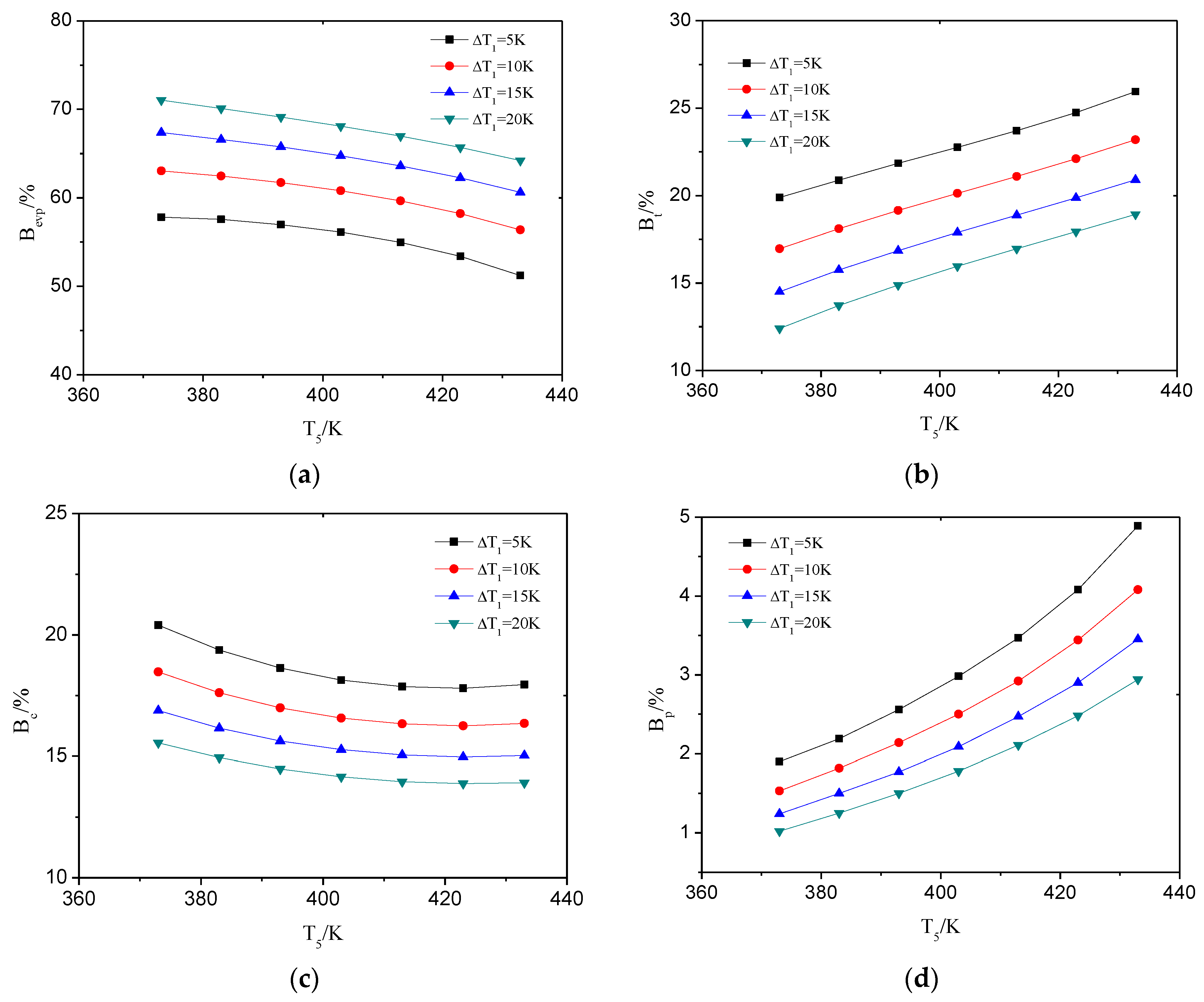
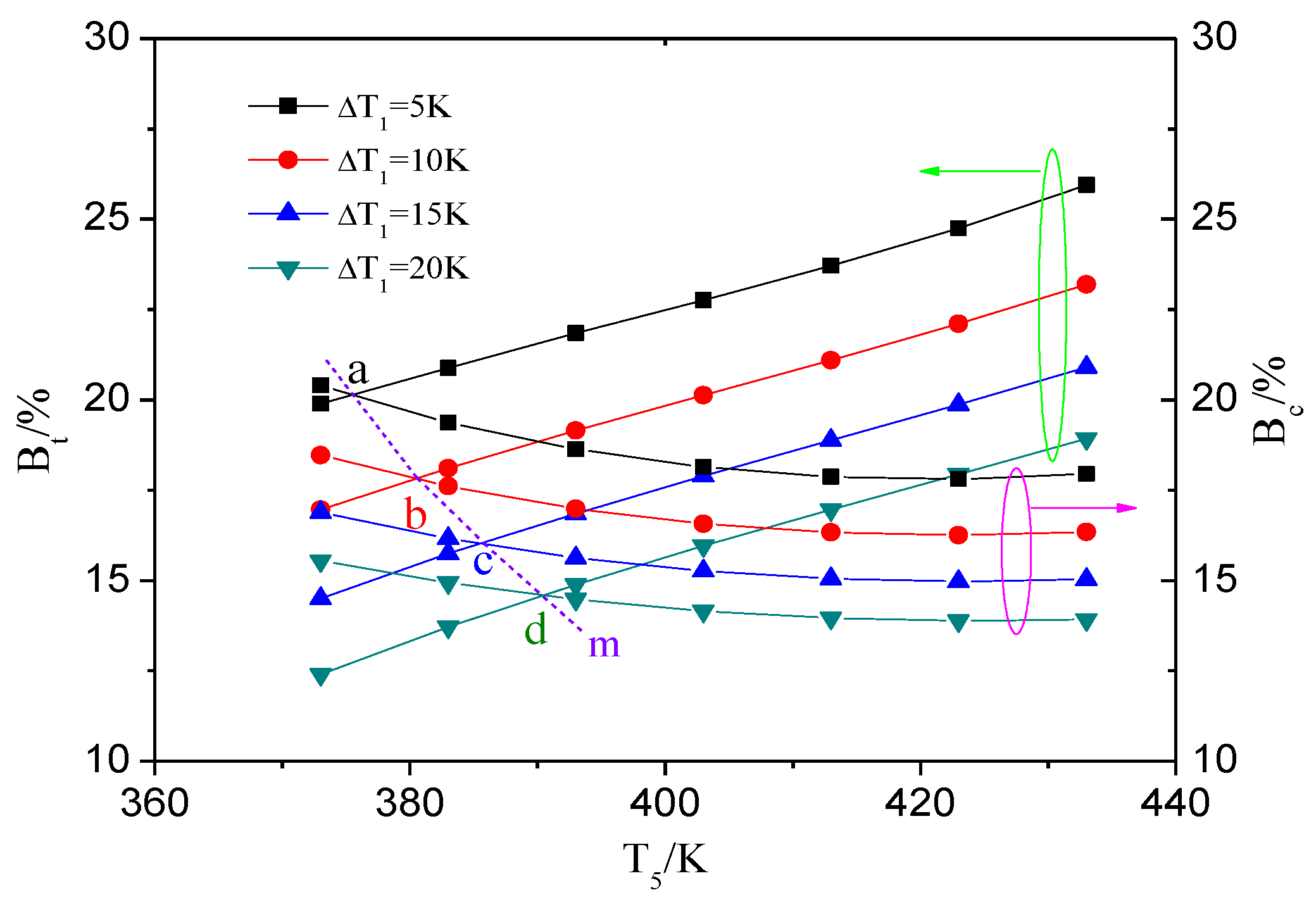
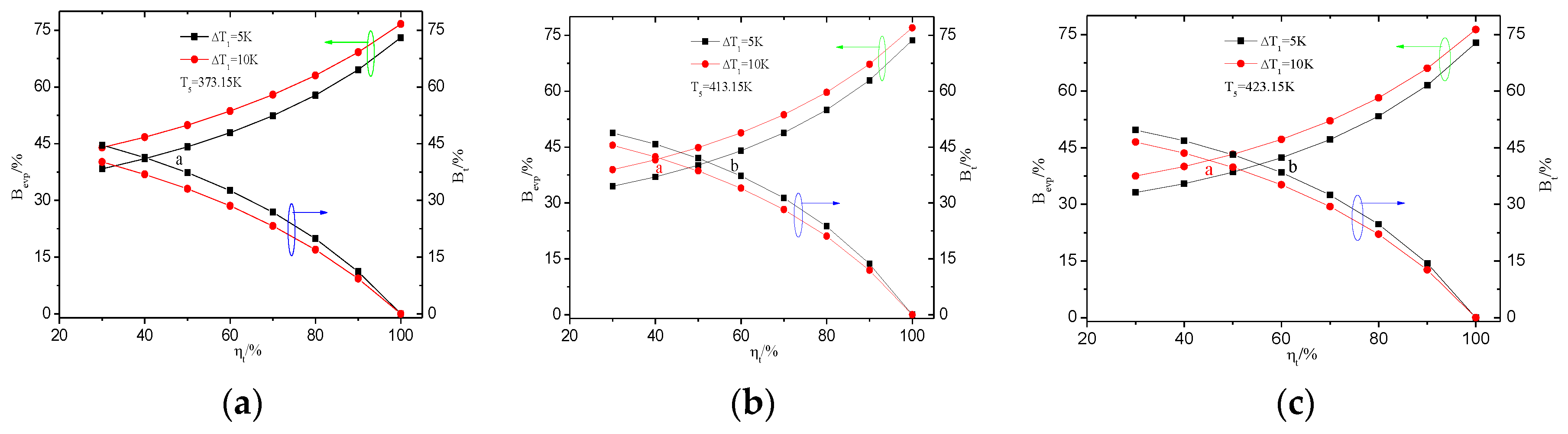
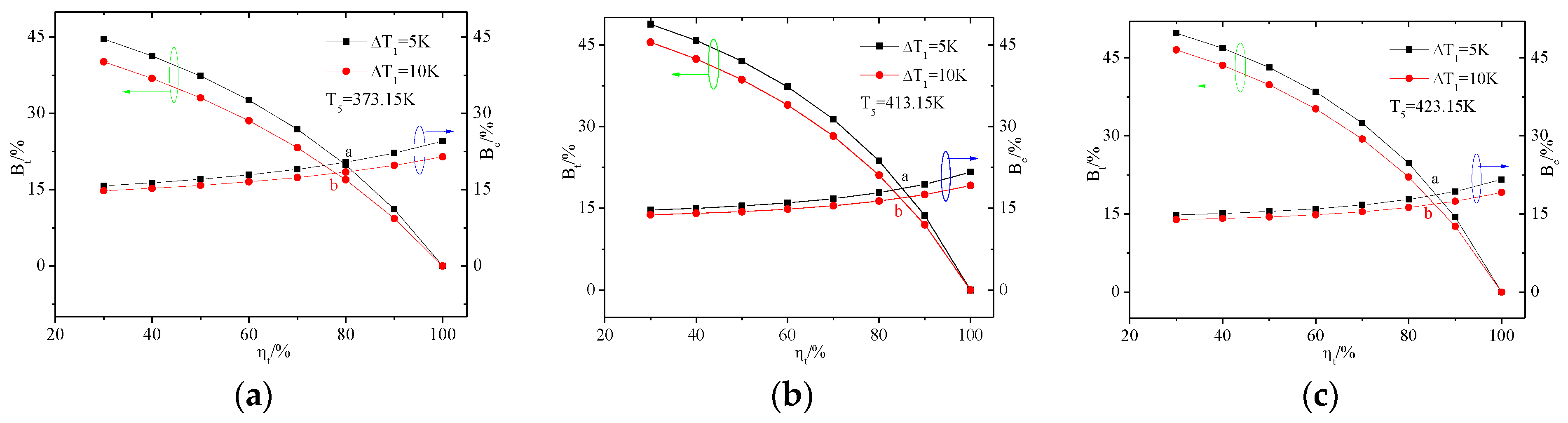
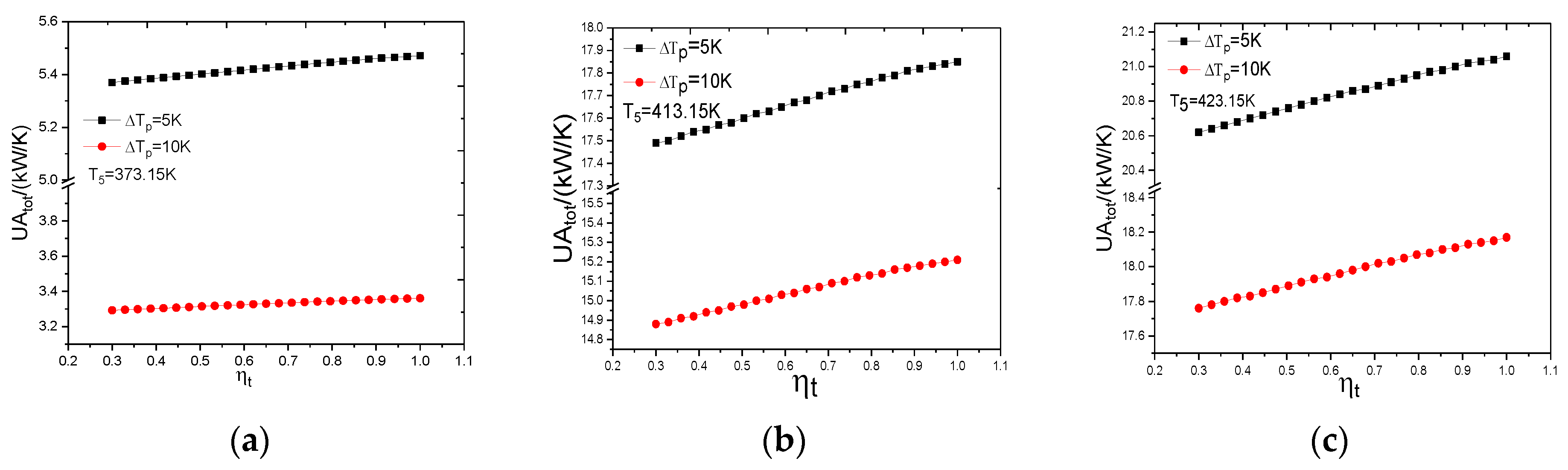
4.1. The Influence of Heat Source Temperature and Evaporator Pinch Point Temperature Difference
4.1.1. The Exergy Loss Distribution of ORC System
4.1.2. The Heat Transfer Capability of ORC System
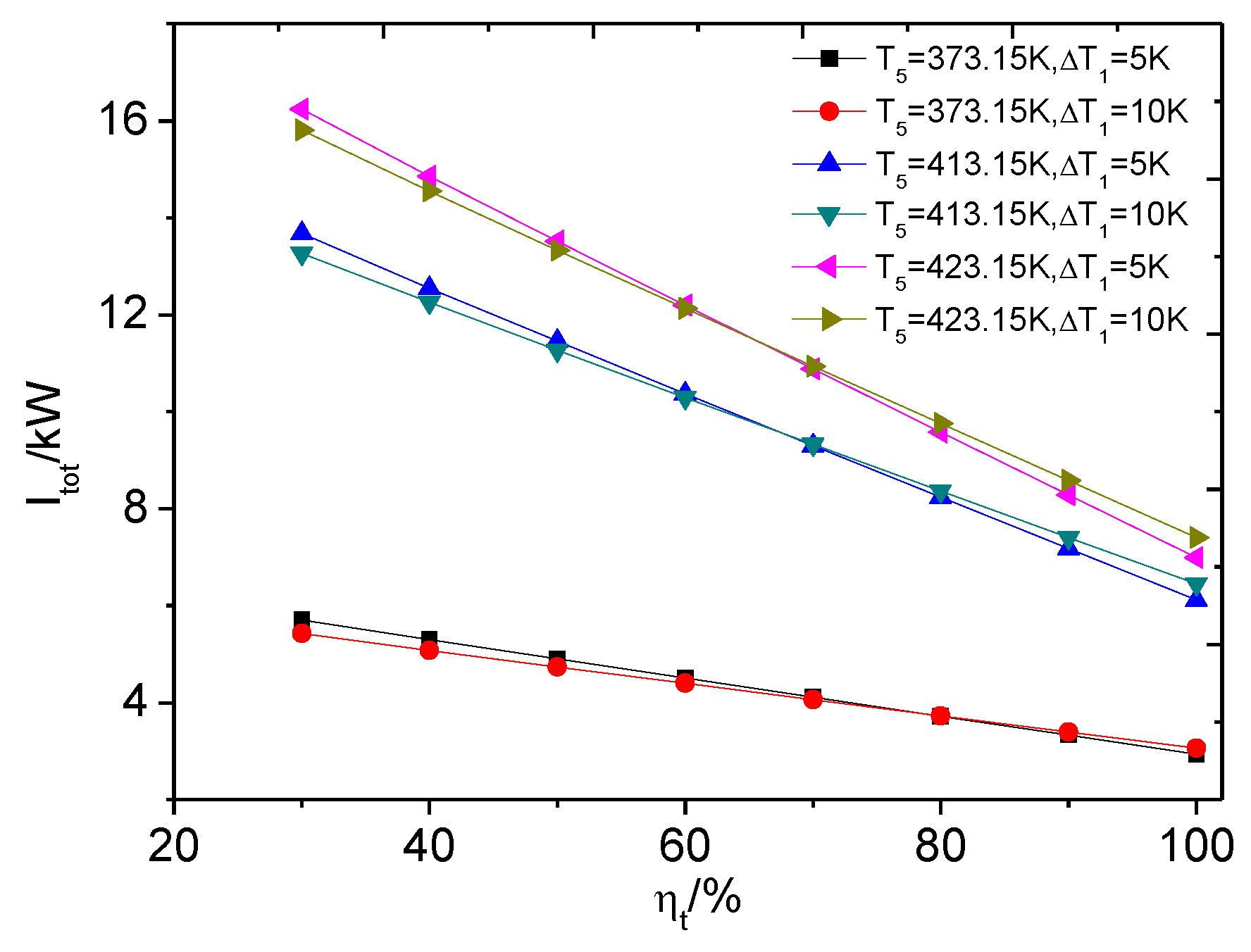
4.2. The Influence of Expander Isentropic Efficiency
4.2.1. The Exergy Loss Distribution of ORC System
4.2.2. The Heat Transfer Capability of ORC System
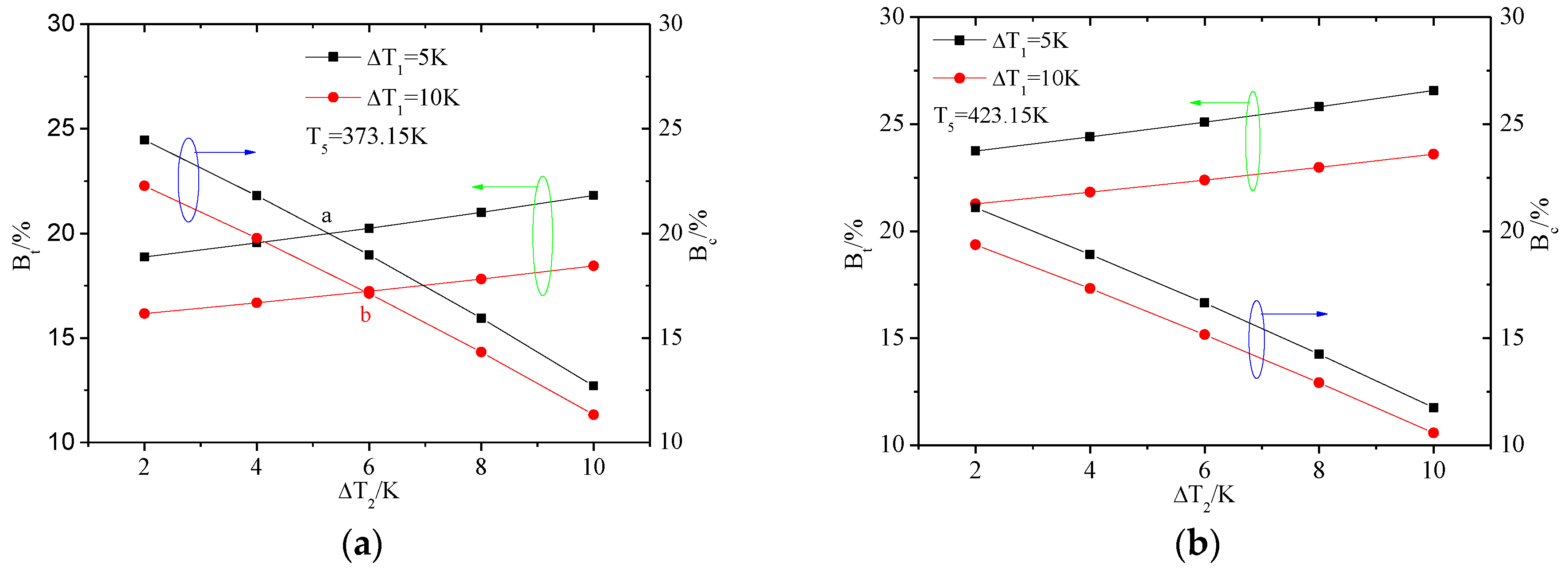
4.3. The Influence of Cooling Water Temperature Rise
4.3.1. The Exergy Loss Distribution
4.3.2. The Heat Transfer Capability of ORC System
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| B | the proportion of exergy loss |
| E | exergy (kW) |
| h | specific enthalpy(kJ·kg−1) |
| exergy loss (kW) | |
| UA | the heat transfer capability (kW/K) |
| mass flow rate (kg s−1) | |
| the heat rate injected and rejected (kW) | |
| s | specific entropy (kJ·kg−1) |
| T | temperature (K) |
| Th | the average temperature of waste heat source (K) |
| Tl | the average temperature of cooling water (K) |
| power output or input (kW) |
Greek symbols
| efficiency (dimensionless) |
Subscripts
| c | condenser |
| evp | evaporator |
| g | generator |
| h | waste heat source |
| net | net |
| p | pump |
| s | isentropic |
| t | expander |
| th | thermal |
| tot | total |
| wf | working fluid |
| 0 | reference state point |
| 1–8 | state points |
| 2s, 4s | stat points for the ideal case |
References
- Tamamoto, T.; Furuhata, T.; Arai, N.; Mori, K. Design and testing of the organic Rankine cycle. Energy 2001, 26, 239–251. [Google Scholar] [CrossRef]
- Liu, B.T.; Chien, K.H.; Wang, C.C. Effect of working fluids on organic Rankine cycle for waste heat recovery. Energy 2004, 29, 1207–1217. [Google Scholar] [CrossRef]
- Wei, D.H.; Lu, X.S.; Lu, Z.; Gu, J.M. Dynamic modeling and simulation of an Organic Rankine Cycle (ORC) system for waste heat recovery. Appl. Therm. Eng. 2008, 28, 1216–1224. [Google Scholar] [CrossRef]
- Roy, J.P.; Mishra, M.K.; Misra, A. Performance analysis of an Organic Rankine Cycle with superheating under different heat source temperature conditions. Appl. Energy 2011, 88, 2995–3004. [Google Scholar] [CrossRef]
- Pierobon, L.; Benato, A.; Scolari, E.; Haglind, F.; Stoppato, A. Waste heat recovery technologies for offshore platforms. Appl. Energy 2014, 136, 228–241. [Google Scholar] [CrossRef]
- Benato, A.; Kærn, M.R.; Pierobon, L.; Stoppato, A.; Haglind, F. Analysis of hot spots in boilers of organic Rankine cycle units during transient operation. Appl. Energy 2015, 151, 119–131. [Google Scholar] [CrossRef]
- Benato, A.; Macor, A. Biogas engine waste heat recovery using organic Rankine cycle. Energies 2017, 10, 327. [Google Scholar] [CrossRef]
- Hung, T.C.; Wang, S.K.; Kuo, C.H.; Pei, B.S.; Tsai, K.F. A study of organic working fluids on system efficiency of an ORC using low-grad energy sources. Energy 2010, 35, 1403–1411. [Google Scholar] [CrossRef]
- Mago, P.J.; Chamra, L.M.; Somayaji, C. Performance analysis of different working fluids for use in organic Rankin cycles. Proc. Inst. Mech. Eng. Part A 2007, 221, 255–264. [Google Scholar] [CrossRef]
- Chen, H.J.; Goswami, D.Y.; Stefanakos, E.K. A review of thermodynamic cycles and working fluids for the conversion of low-grade heat. Renew. Sustain. Energy Rev. 2010, 14, 3059–3067. [Google Scholar] [CrossRef]
- Saleh, B.; Koglbauer, G.; Wendland, M.; Fischer, J. Working fluids for low-temperature organic Rankine cycles. Energy 2007, 32, 1210–1221. [Google Scholar] [CrossRef]
- Lai, N.A.; Wendland, M.; Fischer, J. Working fluids for high-temperature organic Rankine cycles. Energy 2011, 36, 199–211. [Google Scholar] [CrossRef]
- Hung, T.C. Waste heat recovery of organic Rankine cycle using dry fluids. Energy Convers. Manag. 2001, 42, 539–553. [Google Scholar] [CrossRef]
- Wei, D.H.; Lu, X.S.; Lu, Z.; Gu, J.M. Performance analysis and optimization of organic Rankine cycle (ORC) for waste heat recovery. Energy Convers. Manag. 2007, 48, 1113–1119. [Google Scholar] [CrossRef]
- Mago, P.J.; Srinivasan, K.K.; Chamra, L.M.; Somayaji, C. An examination of exergy destruction in organic Rankine cycles. Int. J. Energy Res. 2008, 32, 926–938. [Google Scholar] [CrossRef]
- Tchanche, B.F.; Papadakis, G.; Lambrinos, G. Frangoudakis, A. Fluid selection for a low-temperature solar organic Rankine cycle. Appl. Therm. Eng. 2009, 29, 2468–2476. [Google Scholar] [CrossRef]
- Aijundi, I.H. Effect of dry hydrocarbons and critical point temperature on the efficiencies of organic Rankine cycle. Renew. Energy 2011, 36, 1196–1202. [Google Scholar] [CrossRef]
- Wang, E.H.; Zhang, H.G.; Fan, B.Y.; Ouyang, M.G.; Zhao, Y.; Mu, Q.H. Study of working fluid selection of organic Rankine cycle (ORC) for engine waste heat recovery. Energy 2011, 36, 3406–3418. [Google Scholar] [CrossRef]
- Xu, R.J.; He, Y.L. A vapor injector-based novel regenerative organic Rankine cycle. Appl. Therm. Eng. 2011, 31, 1238–1243. [Google Scholar] [CrossRef]
- Guo, T.; Wang, H.X.; Zhang, S.J. Comparative analysis of natural and conventional working fluids for use in transcriticalRankine cycle using low-temperature geothermal source. Int. J. Energy Res. 2011, 35, 530–544. [Google Scholar] [CrossRef]
- Mago, P.J.; Luck, R. Energetic and exergetic analysis of waste heat recovery from a microturbine using organic Rankine cycles. Int. J. Energy Res. 2012. [Google Scholar] [CrossRef]
- Manolakos, D.; Kosmadakis, G.; Kyritsis, S.; Papadakis, G. Identification of behaviour and evaluation of performance of small scale, low-temperature organic Rankine cycle system coupled with a RO desalination unit. Energy 2009, 34, 767–774. [Google Scholar] [CrossRef]
- Peterson, R.B.; Wang, H.; Herron, T. Performance of small-scale regenerative Rankine power cycle employing a scroll expander. Proc. Inst. Mech. Eng. Part A 2008, 222, 271–282. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhao, L.; Wang, J.L.; Zhang, W.Z.; Zhao, X.Z.; Wu, W. Performance evaluation of a low-temperature solar Rankine cycle system utilizing R245fa. Sol. Energy 2010, 84, 353–364. [Google Scholar] [CrossRef]
- Lemort, V.; Quoilin, S.; Cuevas, C.; Lebrun, J. Testing and modeling a scroll expander integrated into an organic Rankine cycle. Appl. Therm. Eng. 2009, 29, 3094–3102. [Google Scholar] [CrossRef]
- Quoilin, S.; Lemort, V.; Lebrun, J. Experimental study and modeling of an organic Rankine cycle using scroll expander. Appl. Energy 2010, 87, 1260–1268. [Google Scholar] [CrossRef]
- Li, J.; Pei, G.; Li, Y.Z.; Ji, J. Evaluation of external heat loss from a small-scale expander used in organic Rankine cycle. Appl. Therm. Eng. 2011, 31, 2694–2701. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Li, L.; Shu, P. Simulation and experiment research on wide ranging working process of scroll expander driven by compressed air. Appl. Therm. Eng. 2010, 30, 2073–2079. [Google Scholar]
- James, A.M.; Jon, R.J.; Cao, J.; Douglas, K.P.; Richard, N.C. Experimental testing of gerotor and scroll expanders used in, and energetic and exergetic modeling of an organic Rankine cycle. J. Energy Resour. Technol. 2009, 131, 201–208. [Google Scholar]
- Heberle, F.; Brüggemann, D. Exergy based fluid selection for a geothermal Organic Rankine Cycle for combined heat and power generation. Appl. Therm. Eng. 2010, 30, 1326–1332. [Google Scholar] [CrossRef]
- Heberle, F.; Preißinger, M.; Brüggemann, D. Zeotropic mixtures as working fluids in Organic Rankine Cycles for low-enthalpy geothermal resources. Renew. Energy 2012, 37, 364–370. [Google Scholar] [CrossRef]
- Eller, T.; Heberle, F.; Brüggemann, D. Second law analysis of novel working fluid pairs for waste heat recovery by the Kalina cycle. Energy 2017, 119, 188–198. [Google Scholar] [CrossRef]
- Liu, C.; He, C.; Gao, H.; Xu, X.X.; Xu, J.L. The optimal evaporation temperature of subcritical ORC based on second law efficiency for waste heat recovery. Entropy 2012, 14, 491–504. [Google Scholar] [CrossRef]
- Pezzuolo, A.; Benato, A.; Stoppato, A.; Mirandola, A. The ORC-PD: A versatile tool for fluid selection and Organic Rankine Cycle unit design. Energy 2016, 102, 605–620. [Google Scholar] [CrossRef]
- Guo, T.; Wang, H.X.; Zhang, S.J. Fluids and parameters optimization for a novel cogeneration system drivenby low-temperature geothermal sources. Energy 2011, 36, 2639–2649. [Google Scholar] [CrossRef]
- Eyerera, S.; Wielanda, C.; Vandersickela, A.; Spliethoff, H. Experimental study of an ORC (Organic Rankine Cycle) and analysis of R1233zd-E as a drop-in replacement for R245fa for low temperature heat utilization. Energy 2016, 103, 660–671. [Google Scholar] [CrossRef]
- Wielanda, C.; Meinela, D.; Eyerera, S.; Spliethoff, H. Innovative CHP concept for ORC and its benefit compared to conventional concepts. Appl. Energy 2016, 183, 478–490. [Google Scholar] [CrossRef]
- Dai, Y.P.; Wang, J.F.; Lin, G. Parametric optimization and comparative study of organic Rankine cycle (ORC) for low grade waste heat recovery. Energy Convers. Manag. 2009, 50, 576–582. [Google Scholar] [CrossRef]



| Description | Data |
|---|---|
| Waste heat inlet temperature (K) | 373.15–433.15 |
| The mass flow rate of heat source (kg/s) | 1 |
| The evaporator pinch point temperature difference (K) | 5–20 |
| Expander isentropic efficiency (%) | 30–100 |
| Pump isentropic efficiency (%) | 75 |
| Cooling water inlet temperature (K) | 293.15 |
| Cooling water temperature rise (K) | 2–10 |
| Environment temperature (K) | 293.15 |
| Environment pressure (kPa) | 100 |
| Working Fluid | T1 (K) | P1 (kPa) | T2 (K) | P2 (kPa) | T4 (K) | (kg/s) | (kW) | (%) | Data Source |
|---|---|---|---|---|---|---|---|---|---|
| R123 | 356.38 | 530 | 311.59 | 91 | 298.5 | 6.47 | 156.91 | 11.83 | Reference [38] |
| R123 | 356.4 | 531.7 | 312.2 | 91.48 | 298.5 | 6.036 | 147.9 | 11.88 | This paper |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Jiao, Y.; Tian, C.; Wang, Z.; Zhang, Z. The Exergy Loss Distribution and the Heat Transfer Capability in Subcritical Organic Rankine Cycle. Entropy 2017, 19, 256. https://doi.org/10.3390/e19060256
He C, Jiao Y, Tian C, Wang Z, Zhang Z. The Exergy Loss Distribution and the Heat Transfer Capability in Subcritical Organic Rankine Cycle. Entropy. 2017; 19(6):256. https://doi.org/10.3390/e19060256
Chicago/Turabian StyleHe, Chao, Youzhou Jiao, Chaochao Tian, Zhenfeng Wang, and Zhiping Zhang. 2017. "The Exergy Loss Distribution and the Heat Transfer Capability in Subcritical Organic Rankine Cycle" Entropy 19, no. 6: 256. https://doi.org/10.3390/e19060256





