Effects of Fatty Infiltration of the Liver on the Shannon Entropy of Ultrasound Backscattered Signals
Abstract
:1. Introduction
2. Materials and Methods
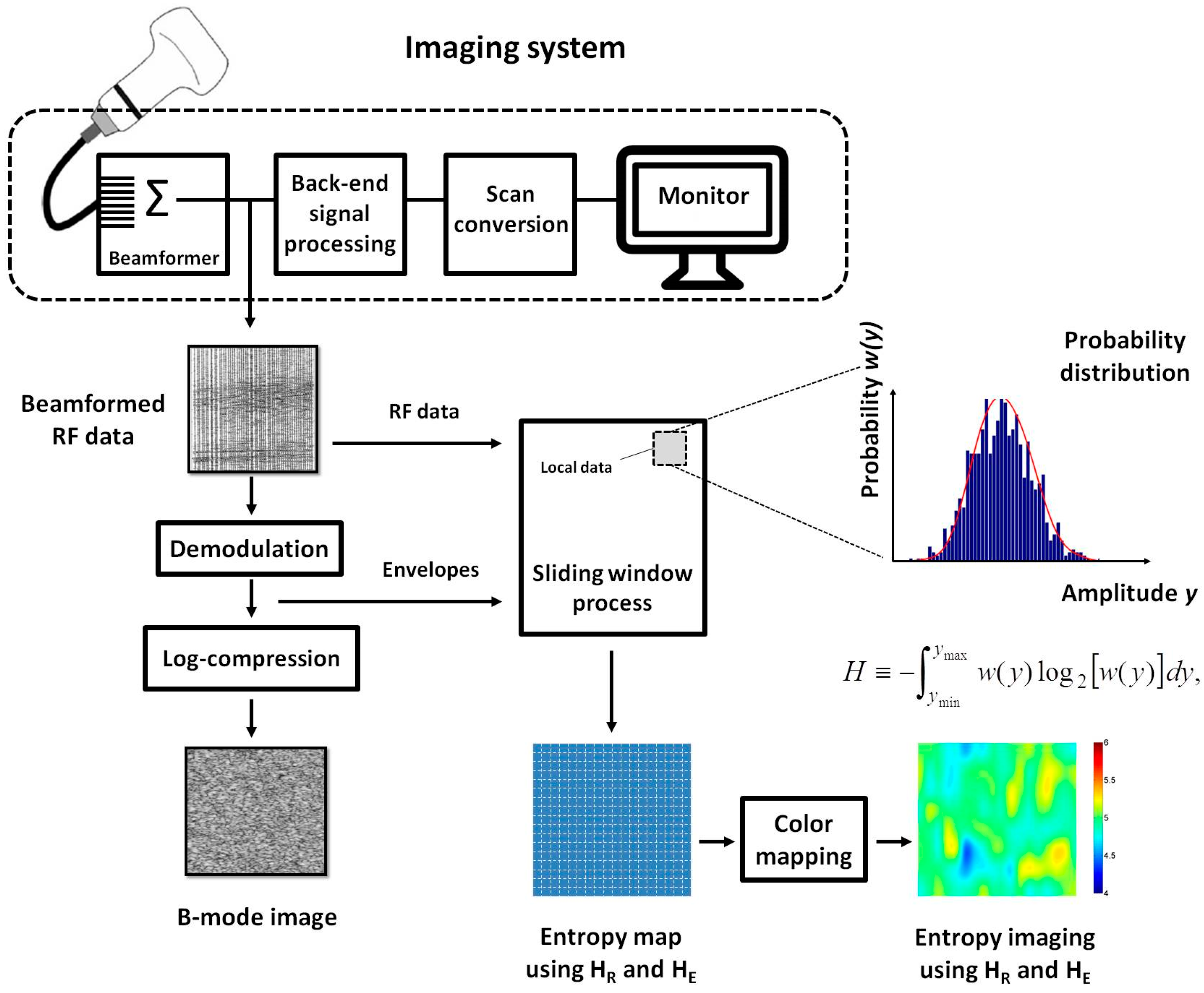
2.1. Entropy Estimation
2.2. Data Collection
2.3. Scoring System of Fatty Liver
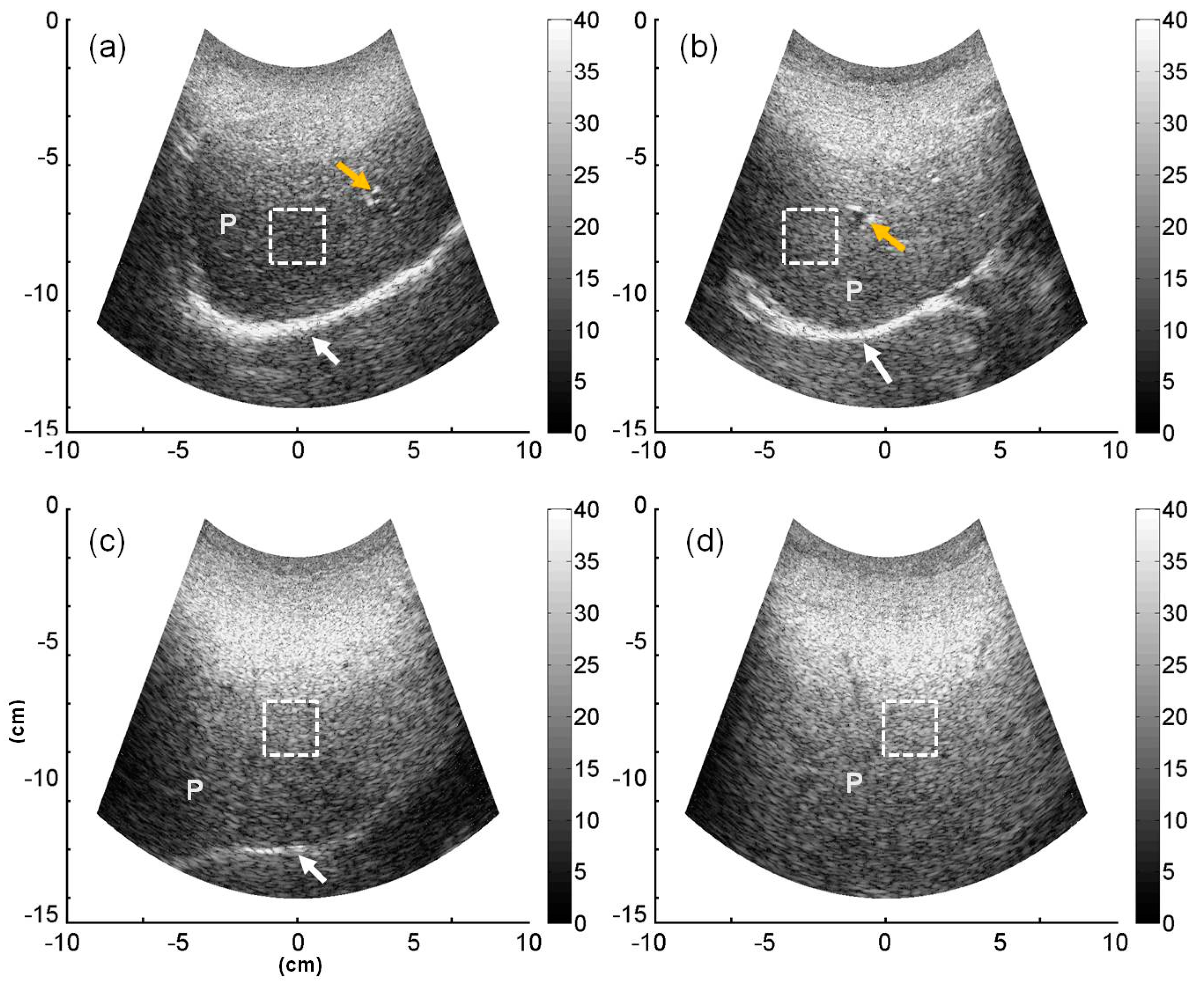
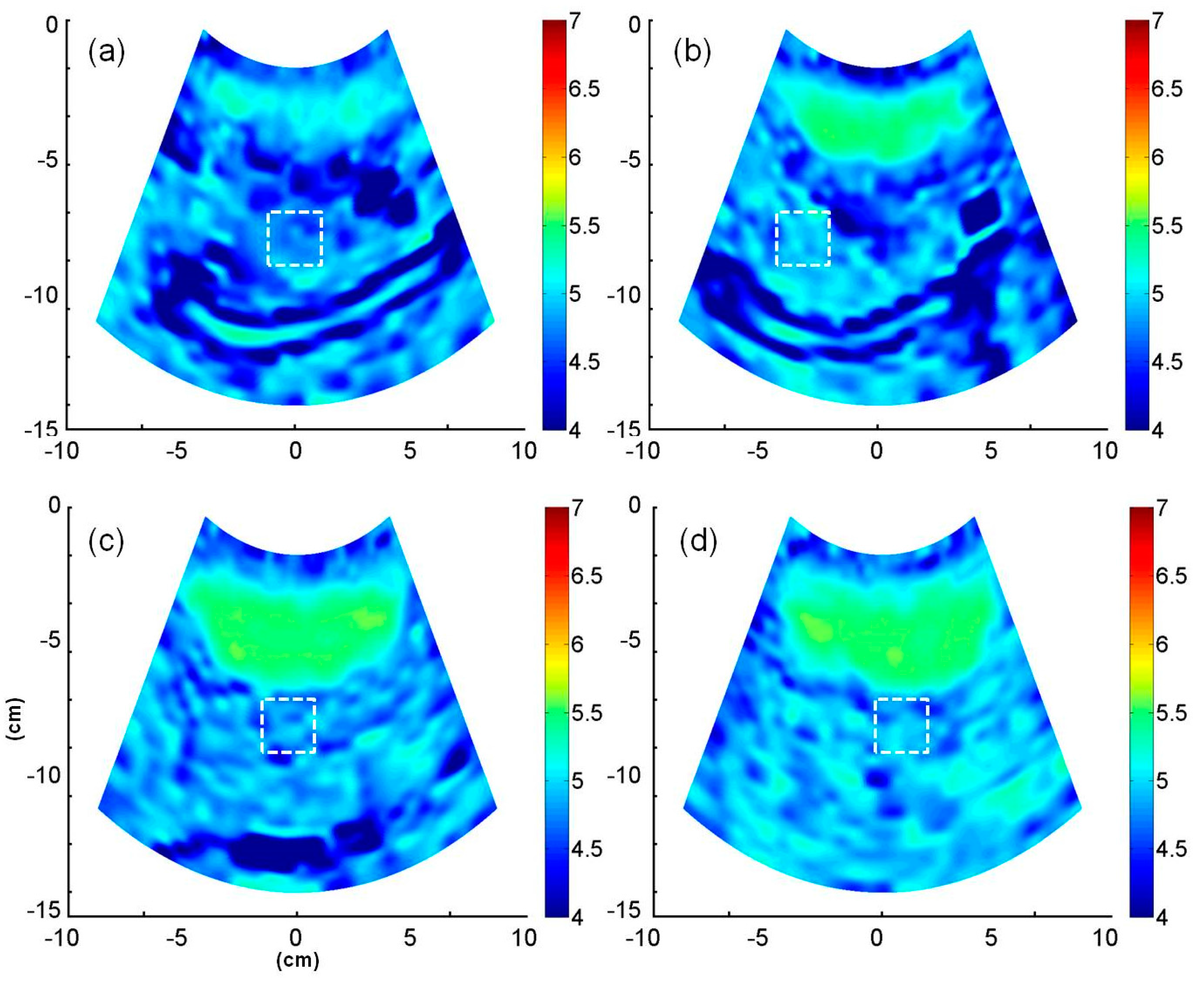
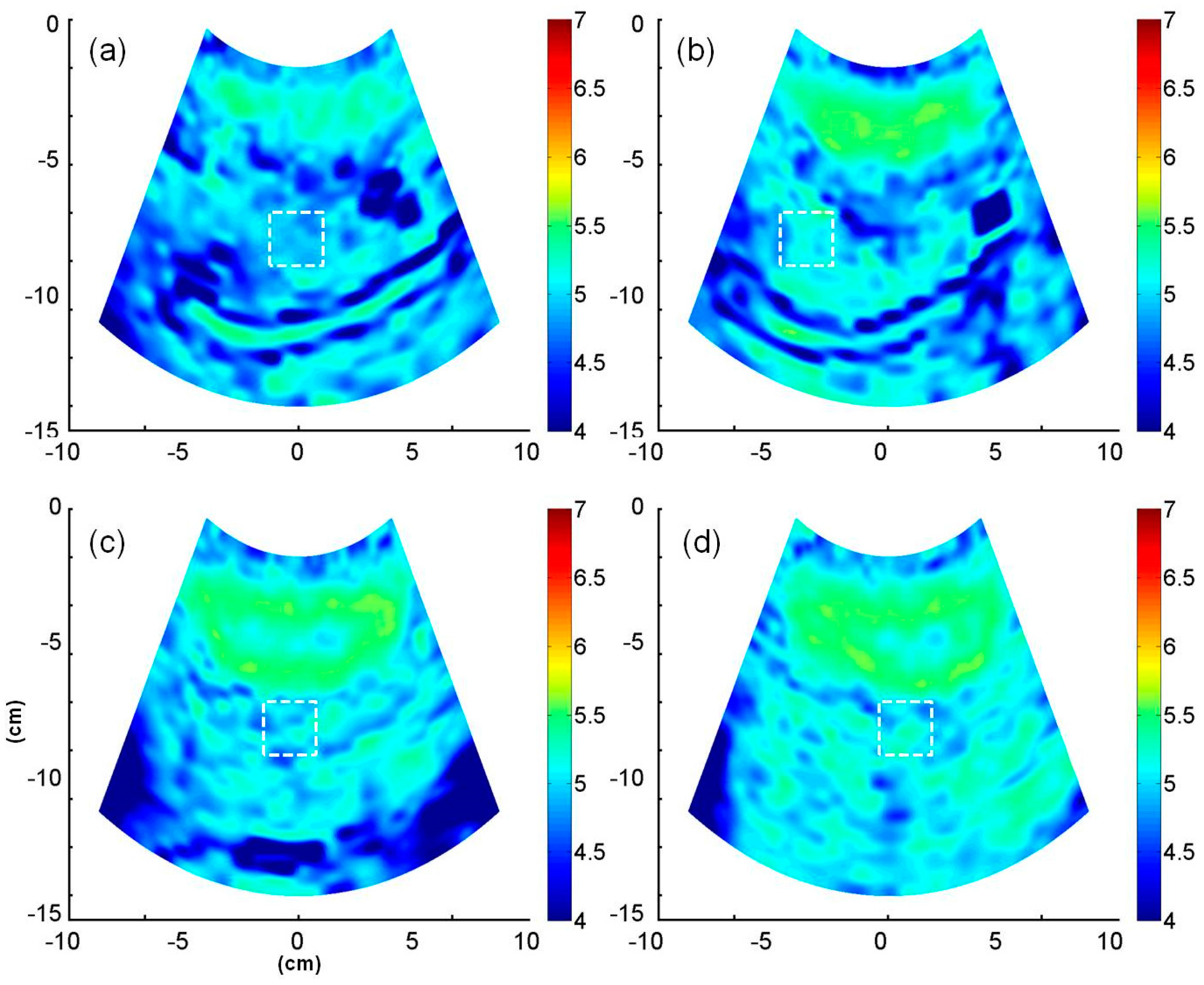
2.4. B-Mode and Entropy Imaging
2.5. Statistical Analysis
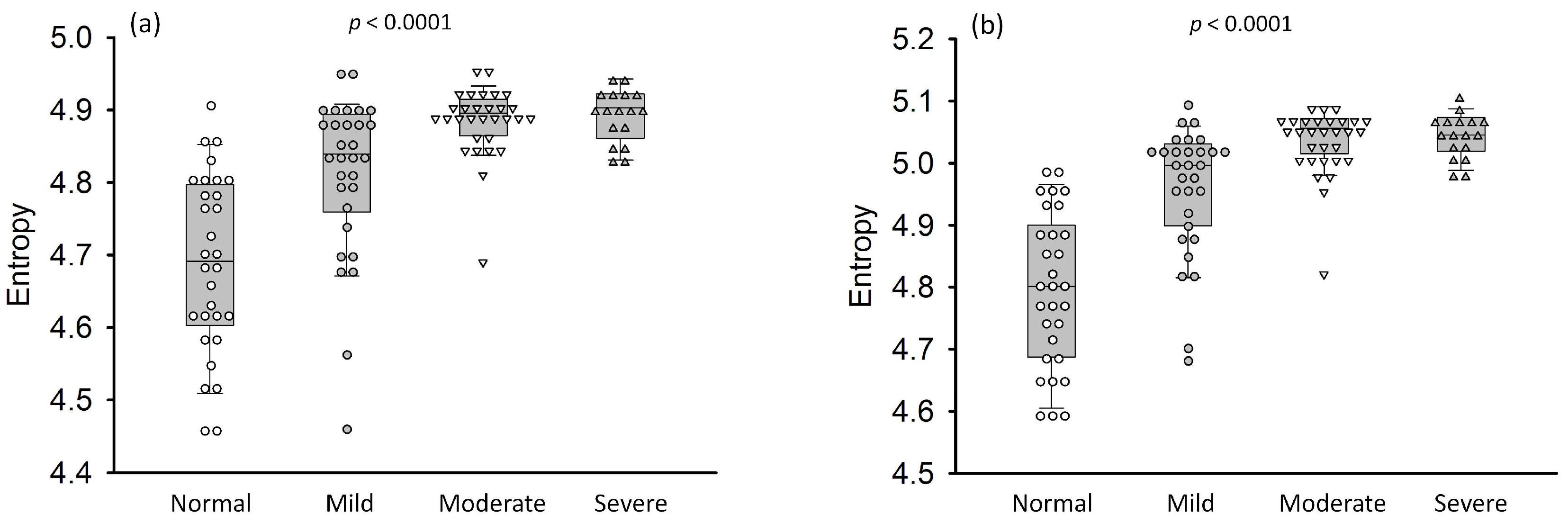
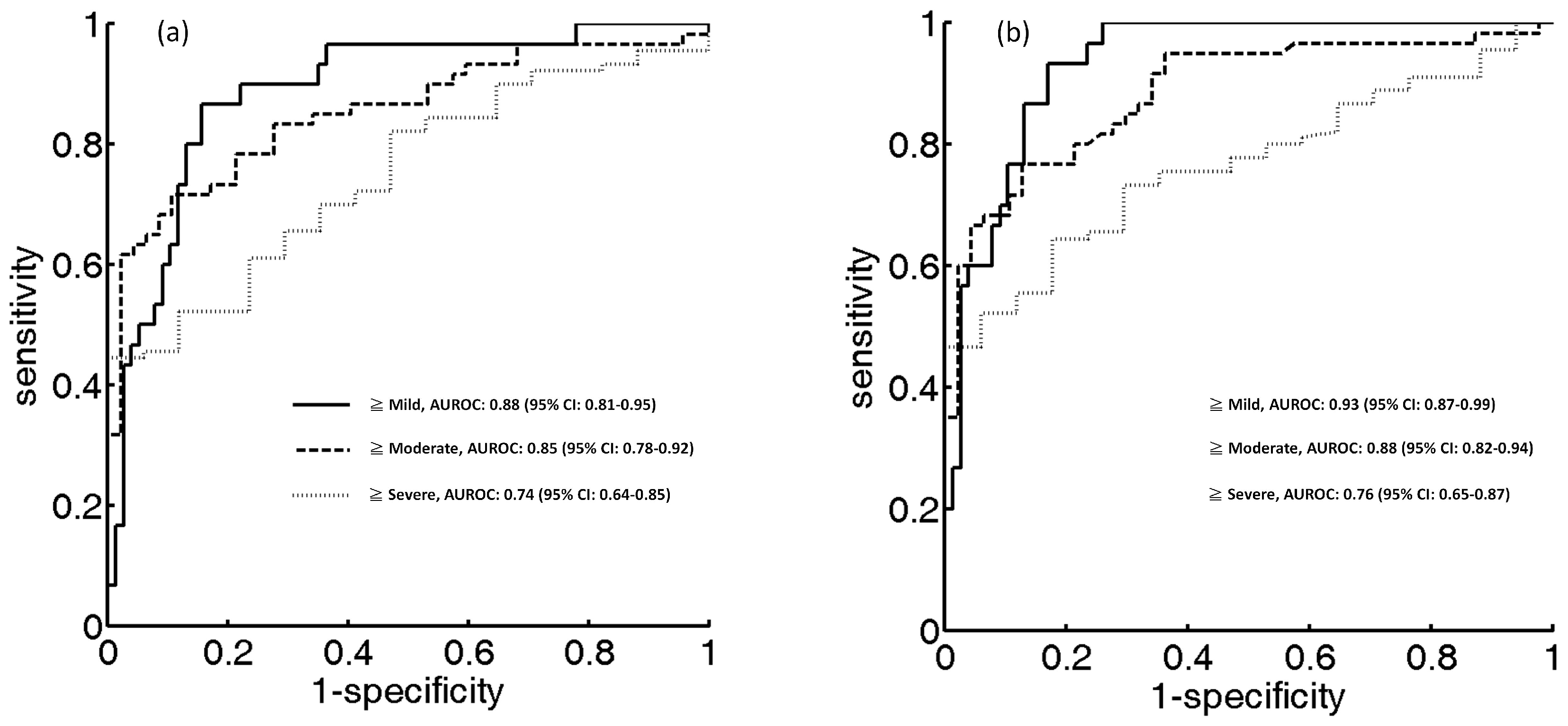
3. Results
4. Discussion
4.1. Significance of the Study
4.2. Effects of Fatty Infiltration on Ultrasound Entropy
4.3. Potential of Entropy Imaging in Evaluating Fatty Liver
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raff, E.J.; Kakati, D.; Bloomer, J.R.; Shoreibah, M.; Rasheed, K.; Singal, A.K. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases. J. Clin. Transl. Hepatol. 2015, 3, 9–16. [Google Scholar] [PubMed]
- Loomba, R.; Abraham, M.; Unalp, A.; Wilson, L.; Lavine, J.; Doo, E.; Bass, N.M. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012, 56, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Heba, E.; Wolfson, T.; Ang, B.; Gamst, A.; Han, A.; Erdman, J.W., Jr.; O’Brien, W.D., Jr.; Andre, M.P.; Sirlin, C.B.; et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin. Gastroenterol. Hepatol. 2015, 13, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoglu, I.; Brunt, E.M. Role of liver biopsy in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 9026–9037. [Google Scholar] [PubMed]
- Wilkins, T.; Tadkod, A.; Hepburn, I.; Schade, R.R. Nonalcoholic fatty liver disease: Diagnosis and management. Am. Fam. Physician 2013, 88, 35–42. [Google Scholar] [PubMed]
- Hui, J.M.; Farrell, G.C.; Kench, J.G.; George, J. High sensitivity C-reactive protein values do not reliably predict the severity of histological changes in NAFLD. Hepatology 2004, 39, 1458–1459. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.; Bhalla, V.; Regal, M.E.E.; A-Kader, H.H. Nonalcoholic fatty liver disease: A comprehensive review of a growing epidemic. World J. Gastroenterol. 2014, 20, 12082–12101. [Google Scholar] [CrossRef] [PubMed]
- Meisamy, S.; Hines, C.D.G.; Hamilton, G.; Sirlin, C.B.; McKenzie, C.A.; Yu, H.; Brittain, J.H.; Reeder, S.B. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: Blinded comparison with MR spectroscopy. Radiology 2011, 258, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, Y.; Kamiyama, N.; Maruyama, K.; Sumino, Y. Real-time ultrasound attenuation imaging of diffuse fatty liver disease. Ultrasound Med. Biol. 2013, 39, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.F.; Zagzebski, J.A.; Lee, F.T. Ultrasound backscatter and attenuation in human liver with diffuse disease. Ultrasound Med. Biol. 1999, 25, 1047–1054. [Google Scholar] [CrossRef]
- Berzigotti, A.; Castera, L. Update on ultrasound imaging of liver fibrosis. J. Hepatol. 2013, 58, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Kamiyama, N.; Shiraki, K.; Takase, K.; Yamaguchi, T.; Hachiya, H. B-mode ultrasound with algorithm based on statistical analysis of signals: Evaluation of liver fibrosis in patients with chronic hepatitis C. AJR Am. J. Roentgenol. 2009, 193, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Layer, G.; Zuna, I.; Lorenz, A.; Zerban, H.; Haberkorn, U.; Bannasch, P.; van Kaick, G.; Rath, U. Computerized ultrasound B-scan texture analysis of experimental diffuse parenchymal liver disease: Correlation with histopathology and tissue composition. J. Clin. Ultrasound 1991, 19, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gaitini, D.; Baruch, Y.; Ghersin, E.; Veitsman, E.; Kerner, H.; Shalem, B.; Yaniv, G.; Sarfaty, C.; Azhari, H. Feasibility study of ultrasonic fatty liver biopsy: Texture vs. attenuation and backscatter. Ultrasound Med. Biol. 2004, 30, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Faust, O.; Molinari, F.; Sree, S.V.; Junnarkar, S.P.; Sudarshan, V. Ultrasound-based tissue characterization and classification of fatty liver disease: A screening and diagnostic paradigm. Knowl. Based Syst. 2015, 75, 66–77. [Google Scholar] [CrossRef]
- Chang, R.F.; Wu, W.J.; Moon, W.K.; Chen, D.R. Automatic ultrasound segmentation and morphology based diagnosis of solid breast tumors. Breast Cancer Res. Treat. 2005, 89, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, G.J.; Liao, B.; Huang, G.L.; Liang, J.Y.; Zhou, L.Y.; Wang, F.; Li, W.; Xie, X.Y.; Wang, W.; et al. Impact Factors and the Optimal Parameter of Acoustic Structure Quantification in the Assessment of Liver Fibrosis. Ultrasound Med. Biol. 2015, 41, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Liao, B.; Liang, J.Y.; Zhou, L.Y.; Wang, F.; Li, W.; Liu, J.Y.; Xie, X.Y.; Lu, M.D.; et al. Assessment of liver fibrosis in chronic hepatitis B using acoustic structure quantification: Quantitative morphological ultrasound. Eur. Radiol. 2016, 26, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Kakisaka, K.; Kamiyama, N.; Oikawa, T.; Onodera, M.; Sawara, K.; Oikawa, K.; Endo, R.; Takikawa, Y.; Suzuki, K. Non-invasive determination of hepatic steatosis by acoustic structure quantification from ultrasound echo amplitude. World J. Gastroenterol. 2012, 18, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Onodera, M. The new non-invasive quantification of hepatic steatosis with morbid obesity by acoustic structure quantification (ASQ) from ultrasound echo amplitude. Ultrasound Med. Biol. 2013, 39. [Google Scholar] [CrossRef]
- Karlas, T.; Berger, J.; Garnov, N.; Lindner, F.; Busse, H.; Linder, N.; Schaudinn, A.; Relke, B.; Chakaroun, R.; Troltzsch, M.; et al. Estimating steatosis and fibrosis: Comparison of acoustic structure quantification with established techniques. World J. Gastroenterol. 2015, 21, 4894–4902. [Google Scholar] [CrossRef] [PubMed]
- Son, J.Y.; Lee, J.Y.; Yi, N.J.; Lee, K.W.; Suh, K.S.; Kim, K.G.; Lee, J.M.; Han, J.K.; Choi, B.I. Hepatic Steatosis: Assessment with Acoustic Structure Quantification of US Imaging. Radiology 2016, 278, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.; Jaspers, N.; Nierhoff, D.; Kuhr, K.; Bowe, A.; Goeser, T.; Michels, G. Acoustic structure quantification ultrasound software proves imprecise in assessing liver fibrosis or cirrhosis in parenchymal liver diseases. Ultrasound Med. Biol. 2014, 40, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Lavarello, R.J.; Kemmerer, J.P.; Miller, R.J.; Oelze, M.L. Ex vivo study of quantitative ultrasound parameters in fatty rabbit livers. Ultrasound Med. Biol. 2012, 38, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.C.; Lee, Y.H.; Jeng, Y.M.; Chen, C.N.; Chang, K.J.; Tsui, P.H. Relationship between ultrasound backscattered statistics and the concentration of fatty droplets in livers: An animal study. PLoS ONE 2013, 8, e63543. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.L.; Tai, D.I.; Ma, H.Y.; Chiang, B.H.; Chen, C.K.; Tsui, P.H. Effects of fatty infiltration in human livers on the backscattered statistics of ultrasound imaging. Proc. Inst. Mech. Eng. H 2015, 229, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Smolikova, R.; Wachowiak, M.P.; Zurada, J.M. An information-theoretic approach to estimating ultrasound backscatter characteristics. Comput. Biol. Med. 2004, 34, 355–370. [Google Scholar] [CrossRef]
- Tsui, P.H.; Huang, C.C.; Sun, L.; Dailey, S.H.; Shung, K.K. Characterization of lamina propria and vocal muscle in human vocal fold tissue by ultrasound Nakagami imaging. Med. Phys. 2011, 38, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, C.C.; Shung, K.K.; Tsui, P.H.; Fang, J.; Ma, H.Y.; Wu, S.; Lin, C.C. Entropic imaging of cataract lens: An in vitro study. PLoS ONE 2014, 9, e96195. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.M. Comments on ‘The effect of logarithmic compression on the estimation of the Nakagami parameter for ultrasonic tissue characterization’. Phys. Med. Biol. 2006, 51, L23–L26. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.H. Ultrasound detection of scatterer concentration by weighted entropy. Entropy 2015, 17, 6598–6616. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Hughes, M.S. Analysis of ultrasonic waveforms using Shannon entropy. IEEE Ultrason. Symp. Proc. 1992, 1, 1205–1209. [Google Scholar]
- Hughes, M.S. Analysis of digitized waveforms using Shannon entropy. J. Acoust. Soc. Am. 1993, 93, 892–906. [Google Scholar] [CrossRef]
- Hughes, M.S. Analysis of digitized waveforms using Shannon entropy. II. High speed algorithms based on Green’s functions. J. Acoust. Soc. Am. 1994, 95, 2582–2588. [Google Scholar] [CrossRef]
- Hughes, M.S.; McCarthy, J.E.; Marsh, J.N.; Arbeit, J.M.; Neumann, R.G.; Fuhrhop, R.W.; Wallace, K.D.; Znidersic, D.R.; Maurizi, B.N.; Baldwin, S.L.; et al. Properties of an entropy-based signal receiver with an application to ultrasonic molecular imaging. J. Acoust. Soc. Am. 2007, 121, 3542–3557. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.S.; McCarthy, J.E.; Marsh, J.N.; Wickline, S.A. Joint entropy of continuously differentiable ultrasonic waveforms. J. Acoust. Soc. Am. 2013, 133, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Yeung, R.W. A First Course in Information Theory; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Chan, D.F.Y.; Li, A.M.; Chu, W.C.W.; Chan, M.H.M.; Wong, E.M.C.; Liu, E.K.H.; Chan, I.H.S.; Yin, J.; Lam, C.W.K.; Fok, T.F.; et al. Hepatic steatosis in obese Chinese children. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Tandra, S.; Yeh, M.M.; Brunt, E.M.; Vuppalanchi, R.; Cummings, O.W.; Unalp-Arida, A.; Wilson, L.A.; Chalasani, N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J. Hepatol. 2011, 55, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.M. A general statistical model for ultrasonic backscattering from tissues. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.H.; Chang, C.C.; Ho, M.C.; Lee, Y.H.; Chen, Y.S.; Chang, C.C.; Huang, N.E.; Wu, Z.H.; Chang, K.J. Use of Nakagami statistics and empirical mode decomposition for ultrasound tissue characterization by a nonfocused transducer. Ultrasound Med. Biol. 2009, 35, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Shung, K.K.; Thieme, G.A. Ultrasonic Scattering in Biological Tissues; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Ma, H.Y.; Zhou, Z.; Wu, S.; Wan, Y.L.; Tsui, P.H. A Computer-aided diagnosis scheme for detection of fatty liver in vivo based on ultrasound kurtosis imaging. J. Med. Syst. 2016, 40. [Google Scholar] [CrossRef] [PubMed]
| US Features | Score | Definition |
|---|---|---|
| Liver echotexture | 0 | Echo level of the liver parenchyma is homogeneous and no difference in contrast between liver parenchyma and kidney parenchyma |
| 1 | Slightly increase in echo pattern of the liver | |
| 2 | Intermediate between score 1 and 3 | |
| 3 | Gross discrepancy of the increased hepatic to renal cortical echogenicity | |
| Echo penetration and visibility of diaphragm | 0 | Liver structure is clearly defined from the surface to the diaphragm. The outline of the diaphragm is clearly visualized |
| 1 | Mild attenuation of sound beam through the liver | |
| 2 | Intermediate between score 1 and 3 | |
| 3 | Marked attenuation of sound beam through the liver. The diaphragm is not visualized | |
| Clarity of liver vessel structures (portal vein) | 0 | Vessel wall and lumen of the vessel can be clearly visualized |
| 1 | Slight decreased definition of portal venule walls | |
| 2 | Intermediate between score 1 and 3 | |
| 3 | Only the main portal walls can be visualized with absence of all smaller portal venule walls |
| Parameter | ≥Mild | ≥Moderate | ≥Severe |
|---|---|---|---|
| Cutoff value of HR | 4.81 | 4.83 | 4.88 |
| Sensitivity, % | 84.42 | 89.36 | 70.59 |
| Specificity, % | 86.67 | 70.00 | 64.44 |
| Accuracy, % | 85.04 | 79.43 | 66.35 |
| LR+ | 6.33 | 2.97 | 1.98 |
| LR− | 0.17 | 0.15 | 0.45 |
| PPV, % | 68.42 | 89.58 | 92.18 |
| NPV, % | 94.20 | 71.18 | 27.90 |
| AUC (95% CI) | 0.88 (0.81–0.95) | 0.85 (0.78–0.92) | 0.74 (0.64–0.85) |
| Parameter | ≥Mild | ≥Moderate | ≥Severe |
|---|---|---|---|
| Cutoff value of HE | 4.96 | 4.99 | 5.01 |
| Sensitivity, % | 93.33 | 76.66 | 64.44 |
| Specificity, % | 83.11 | 87.23 | 82.35 |
| Accuracy, % | 86.00 | 81.30 | 67.28 |
| LR+ | 5.52 | 6.00 | 3.65 |
| LR− | 0.08 | 0.26 | 0.43 |
| PPV, % | 68.29 | 88.46 | 95.08 |
| NPV, % | 96.96 | 74.54 | 30.43 |
| AUC (95% CI) | 0.93 (0.87–0.99) | 0.88 (0.82–0.94) | 0.76 (0.65–0.87) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsui, P.-H.; Wan, Y.-L. Effects of Fatty Infiltration of the Liver on the Shannon Entropy of Ultrasound Backscattered Signals. Entropy 2016, 18, 341. https://doi.org/10.3390/e18090341
Tsui P-H, Wan Y-L. Effects of Fatty Infiltration of the Liver on the Shannon Entropy of Ultrasound Backscattered Signals. Entropy. 2016; 18(9):341. https://doi.org/10.3390/e18090341
Chicago/Turabian StyleTsui, Po-Hsiang, and Yung-Liang Wan. 2016. "Effects of Fatty Infiltration of the Liver on the Shannon Entropy of Ultrasound Backscattered Signals" Entropy 18, no. 9: 341. https://doi.org/10.3390/e18090341






