Wavelet Entropy as a Measure of Ventricular Beat Suppression from the Electrocardiogram in Atrial Fibrillation
Abstract
:1. Introduction
2. Methods
2.1. Ventricular Activity Suppression by Average Beat Subtraction
2.2. Wavelet Analysis for Identifying Residual Ventricular Activity
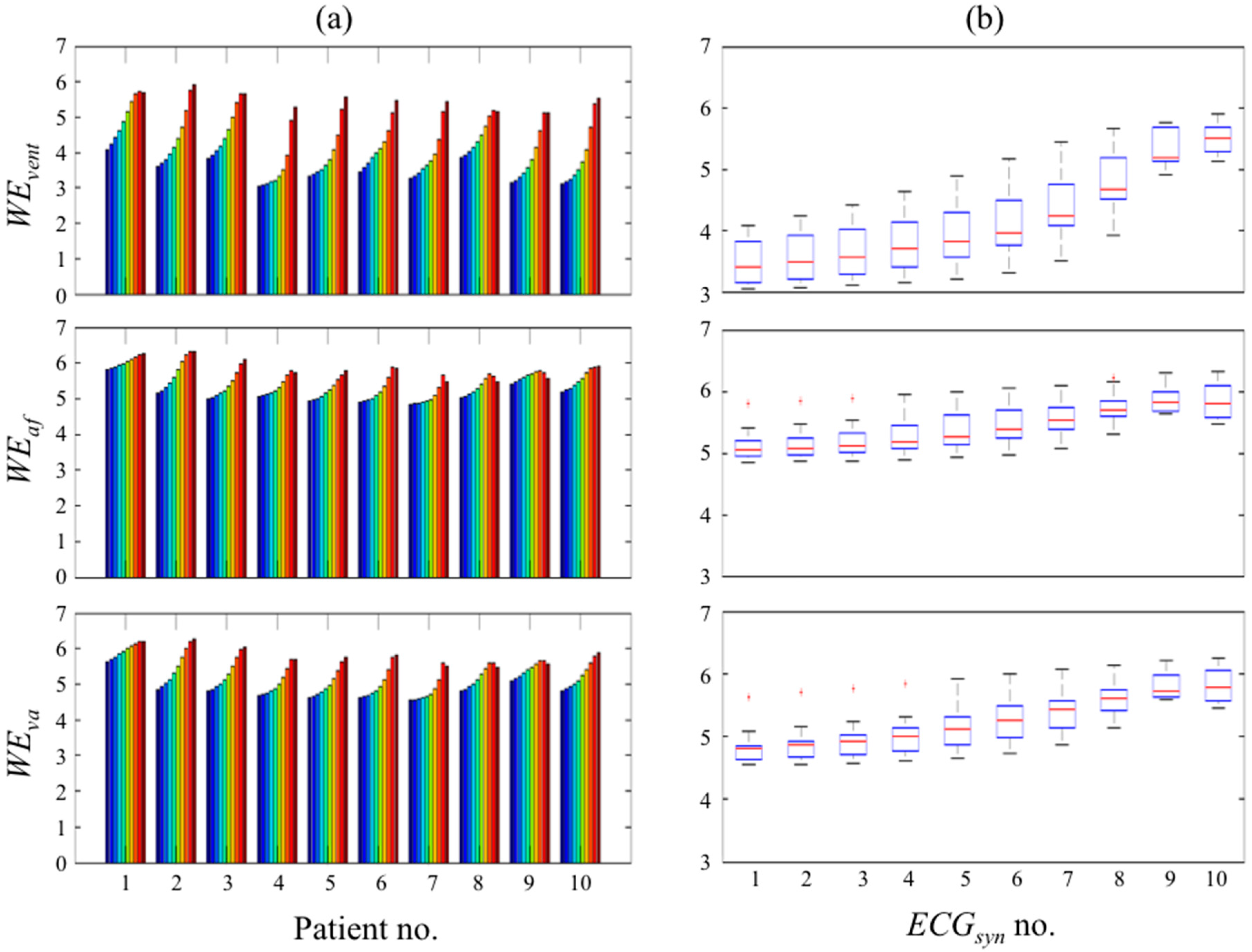
2.3. Wavelet Entropy Measure of Residual Ventricular Activity
- (i)
- Ventricular scale band wavelet entropy (WEvent) defined as the minimum wavelet entropy at scales corresponding to predominantly ventricular activity. Since the ventricular activity, particularly the QRS complex, contains higher frequency components than the f-wave, the frequency range 12.5 to 50 Hz with corresponding scales for the “Gaus4” wavelet of 20 to 5 respectively was used in this study. Motivation for choosing the minimum entropy in this waveband was the observation that the minimum entropy is highly influenced by the degree of QRS residual.
- (ii)
- Atrial scale band wavelet entropy (WEaf) defined as the average wavelet entropy across scales corresponding to predominantly the f-wave. The f-wave has frequencies typically in the range 3 to 10 Hz and the corresponding scales for the “Gaus4” wavelet are 80 to 25 respectively for the sample rate used in this study.
- (iii)
- Wide scale ventricular and atrial band wavelet entropy (WEva) defined as the average wavelet entropy across scales containing both the above atrial and ventricular scale bands specifically 5 (minimum scale of WEvent) to 80 (maximum scale of WEaf). This serves to define a single index for quantifying the quality of ventricular suppression taking account of both atrial and ventricular scale wavelet entropies.
3. Experimental Studies and Discussion
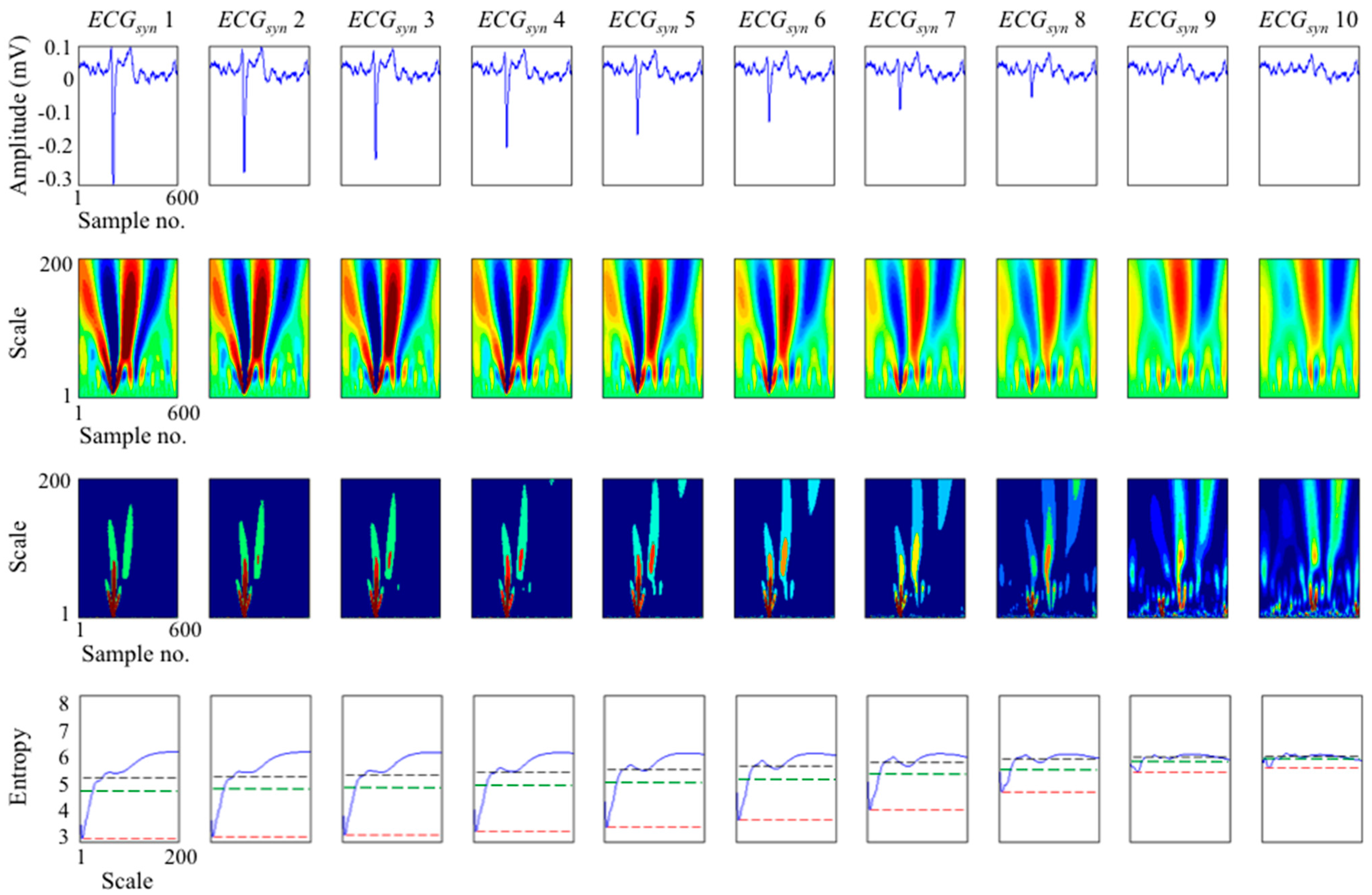
3.1. Study on Synthesised ECGs
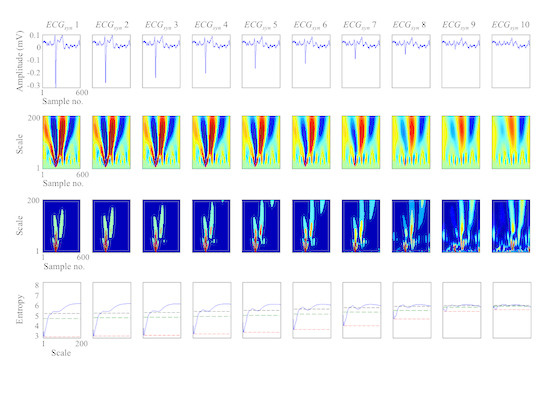
3.2. Study on Real ECGs with ABS Ventricular Suppression
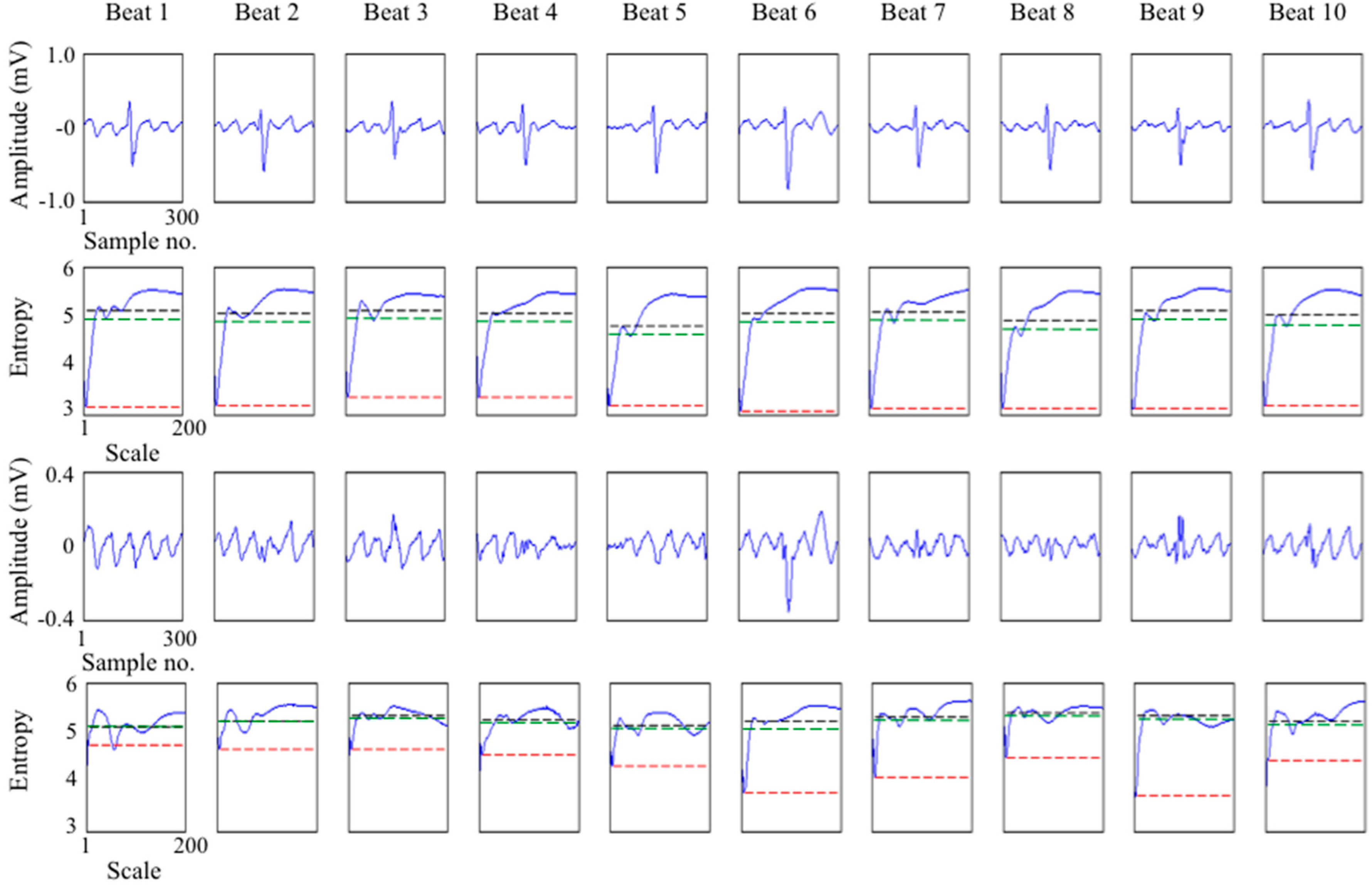

3.3. Application to Multi-Lead ECG
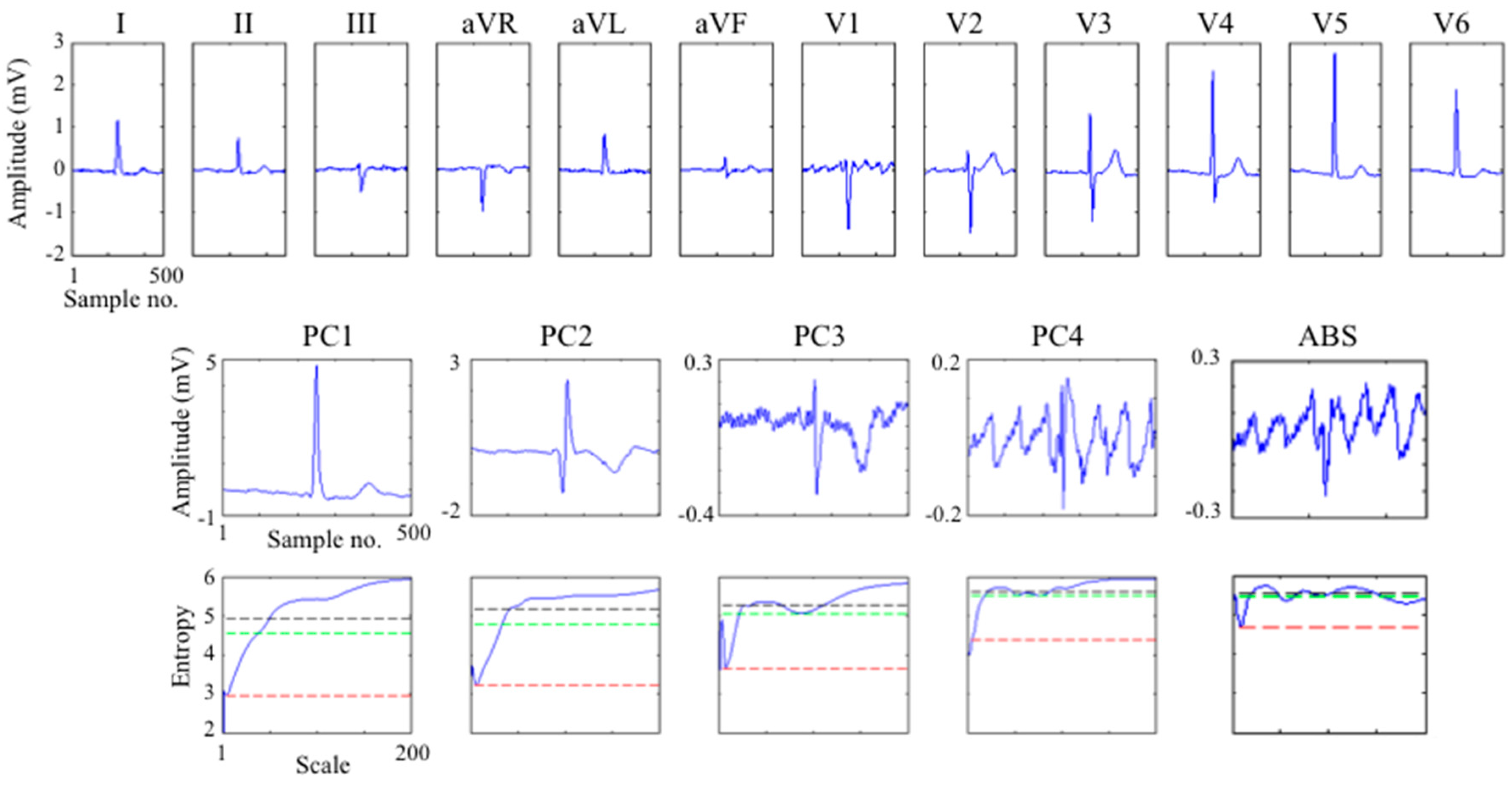
3.4. Comparison to Other Measures of the Effectiveness of Ventricular Beat Suppression

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2014, 64. [Google Scholar] [CrossRef]
- Stewart, S.; Murphy, N.; Walker, A.; McGuire, A.; McMurray, J.J.V. Cost of an emerging epidemic: An economic analysis of atrial fibrillation in the UK. Heart 2004, 90, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Husser, D.; Stridh, M.; Sörnmo, L.; Majic, M.; Klein, H.U.; Olsson, S.B. Frequency measures obtained from the surface electrocardiogram in atrial fibrillation research and clinical decision-making. J. Cardiovasc. Electrophysiol. 2003, 14. [Google Scholar] [CrossRef]
- Ng, J.; Kadish, A.H.; Goldberger, J.J. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm 2006, 3, 1295–1305. [Google Scholar] [CrossRef]
- Guillem, M.S.; Climent, A.M.; Castells, F.; Husser, D.; Millet, J.; Arya, A.; Piorkowski, C.; Bollmann, A. Noninvasive mapping of human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, P.; de la Salud Guillem, M.; Climent, A.M.; Millet, J.; Zarzoso, V.; Castells, F.; Meste, O. Noninvasive assessment of the complexity and stationarity of the atrial wavefront patterns during atrial fibrillation. IEEE Trans. Biomed. Eng. 2010, 57, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Raine, D.; Langley, P.; Murray, A.; Dunuwille, A.; Bourke, J.P. Surface atrial frequency analysis in patients with atrial fibrillation: A tool for evaluating the effects of intervention. J. Cardiovasc. Electrophysiol. 2004, 15, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Husser, D.; Mainardi, L.; Lombardi, F.; Langley, P.; Murray, A.; Rieta, J.J.; Millet, J.; Olsson, S.B.; Stridh, M.; et al. Analysis of surface electrocardiograms in atrial fibrillation: Techniques, research, and clinical applications. Europace 2006, 8, 911–926. [Google Scholar] [PubMed]
- Alcaraz, R.; Rieta, J.J. A non-invasive method to predict electrical cardioversion outcome of persistent atrial fibrillation. Med. Biol. Eng. Comput. 2008, 46, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Meo, M.; Zarzoso, V.; Meste, O.; Latcu, D.G.; Saoudi, N. Catheter ablation outcome prediction in persistent atrial fibrillation using weighted principal component analysis. Biomed. Signal Process. Control 2013, 8, 958–968. [Google Scholar] [CrossRef]
- Alcaraz, R.; Rieta, J.J. Sample entropy of the main atrial wave predicts spontaneous termination of paroxysmal atrial fibrillation. Med. Eng. Phys. 2009, 31, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.; Pehrson, S.; Ingemansson, M.; Sörnmo, L.; Johansson, R.; Sandhall, L.; Sunemark, M.; Smideberg, B.; Olsson, C.; Olsson, S.B. Non-invasive assessment of the atrial cycle length during atrial fibrillation in man: introducing, validating and illustrating a new ECG method. Cardiovasc. Res. 1998, 38, 69–81. [Google Scholar] [CrossRef]
- Stridh, M.; Sörnmo, L. Spatiotemporal QRST cancellation techniques for analysis of atrial fibrillation. IEEE Trans. Biomed. Eng. 2001, 48, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, R.; Rieta, J.J. Adaptive singular value cancelation of ventricular activity in single-lead atrial fibrillation electrocardiograms. Physiol. Meas. 2008, 29, 1351–1369. [Google Scholar] [CrossRef] [PubMed]
- Langley, P.; Bourke, J.P.; Murray, A. Frequency analysis of atrial fibrillation. In Proceedings of Computers in Cardiology, Cambridge, MA, USA, 24–27 September 2000; pp. 65–68.
- Rieta, J.J.; Castells, F.; Sánchez, C.; Zarzoso, V.; Millet, J. Atrial activity extraction for atrial fibrillation analysis using blind source separation. IEEE Trans. Biomed. Eng. 2004, 51, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, L.; Sörnmo, L.; Cerutti, S. Understanding atrial fibrillation: the signal processing contribution, part I; Morgan & Claypool Publisher: San Rafael, CA, USA, 2008. [Google Scholar]
- Mateo, J.; Rieta, J.J. Radial basis function neural networks applied to efficient QRST cancellation in atrial fibrillation. Comput. Biol. Med. 2013, 43, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Petrėnas, A.; Marozas, V.; Sörnmo, L.; Lukosevicius, A. An echo state neural network for QRST cancellation during atrial fibrillation. IEEE Trans. Biomed. Eng. 2012, 59, 2950–2957. [Google Scholar] [PubMed]
- Slocum, J.; Sahakian, A.; Swiryn, S. Diagnosis of atrial fibrillation from surface electrocardiograms based on computer-detected atrial activity. J. Electrocardiol. 1992, 25. [Google Scholar] [CrossRef]
- Xi, Q.; Sahakian, A.V.; Swiryn, S. The effect of QRS cancellation on atrial fibrillatory wave signal characteristics in the surface electrocardiogram. J. Electrocardiol. 2003, 36, 243–249. [Google Scholar] [CrossRef]
- Platonov, P.G.; Corino, V.D.; Seifert, M.; Holmqvist, F.; Sörnmo, L. Atrial fibrillatory rate in the clinical context: Natural course and prediction of intervention outcome. Europace 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Langley, P.; Stridh, M.; Rieta, J.J.; Sörnmo, L.; Millet-Roig, J.; Murray, A. Comparison of atrial rhythm extraction techniques for the estimation of the main atrial frequency from the 12-lead electrocardiogram in atrial fibrillation. In Proceedings of Computers in Cardiology, Memphis, TN, USA, 22–25 September 2002; pp. 29–32.
- Langley, P.; Rieta, J.J.; Stridh, M.; Millet, J.; Sörnmo, L.; Murray, A. Comparison of atrial signal extraction algorithms in 12-lead ECGs with atrial fibrillation. IEEE Trans. Biomed. Eng. 2006, 53, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Petrutiu, S.; Ng, J.; Nijm, G.M.; Al-Angari, H.; Swiryn, S.; Sahakian, A.V. Atrial fibrillation and waveform characterization. Eng. Med. Biol. Mag. 2006, 25, 24–30. [Google Scholar] [CrossRef]
- Goodfellow, J.; Escalona, O.J.; Walsh, P.R.; Kodoth, V.; Manoharan, G. Estimation of atrial fibrillatory frequency by spectral subtraction of wavelet denoised ECG in patients with atrial fibrillation. In Proceedings of Computing in Cardiology, Cambridge, MA, USA, 7–10 September 2014; pp. 329–332.
- Addison, P.S. Wavelet transforms and the ECG: A review. Physiol. Meas. 2005, 26. [Google Scholar] [CrossRef] [PubMed]
- Lemay, M.; Jacquemet, V.; Forclaz, A.; Vesin, J.M. Spatiotemporal QRST cancellation method using separate QRS and T-waves templates. In Proceedings of Computers in Cardiology, Lyon, France, 25–28 September 2005; pp. 611–614.
- Sello, S. Wavelet entropy and the multi-peaked structure of solar cycle maximum. New Astron. 2003, 8, 105–117. [Google Scholar] [CrossRef]
- Rosso, O.A.; Blanco, S.; Yordanova, J.; Kolev, V.; Figliola, A.; Schürmann, M.; Başar, E. Wavelet entropy: A new tool for analysis of short duration brain electrical signals. J. Neurosci. Methods 2001, 105, 65–75. [Google Scholar] [CrossRef]
- Watson, J.N.; Uchaipichat, N.; Addison, P.S.; Clegg, G.R.; Robertson, C.E.; Eftestol, T.; Steen, P.A. Improved prediction of defibrillation success for out-of-hospital VF cardiac arrest using wavelet transform methods. Resuscitation 2004, 63, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Raine, D.; Langley, P.; Shepherd, E.; Lord, S.; Murray, S.; Murray, A.; Bourke, J.P. Principal component analysis of atrial fibrillation: Inclusion of posterior ECG leads does not improve correlation with left atrial activity. Med. Eng. Phys. 2015, 37, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, M.H.; Shin, D.G.; Lee, K.J. Event synchronous adaptive filter based atrial activity estimation in single-lead atrial fibrillation electrocardiograms. Med. Biol. Eng. Comput. 2012, 50, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Lemay, M.; Vesin, J.M.; van Oosterom, A.; Jacquemet, V.; Kappenberger, L. Cancellation of ventricular activity in the ECG: Evaluation of novel and existing methods. IEEE Trans. Biomed. Eng. 2007, 54, 542–546. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langley, P. Wavelet Entropy as a Measure of Ventricular Beat Suppression from the Electrocardiogram in Atrial Fibrillation. Entropy 2015, 17, 6397-6411. https://doi.org/10.3390/e17096397
Langley P. Wavelet Entropy as a Measure of Ventricular Beat Suppression from the Electrocardiogram in Atrial Fibrillation. Entropy. 2015; 17(9):6397-6411. https://doi.org/10.3390/e17096397
Chicago/Turabian StyleLangley, Philip. 2015. "Wavelet Entropy as a Measure of Ventricular Beat Suppression from the Electrocardiogram in Atrial Fibrillation" Entropy 17, no. 9: 6397-6411. https://doi.org/10.3390/e17096397