Ultrasound Detection of Scatterer Concentration by Weighted Entropy
Abstract
:1. Introduction
2. Theoretical Background
2.1. Entropy
2.2. Weighted Entropy
2.3. Ultrasound Weighted Entropy
3. Materials and Methods
3.1. Simulation Model
3.2. Weighted Entropy Estimation of Ultrasound Data
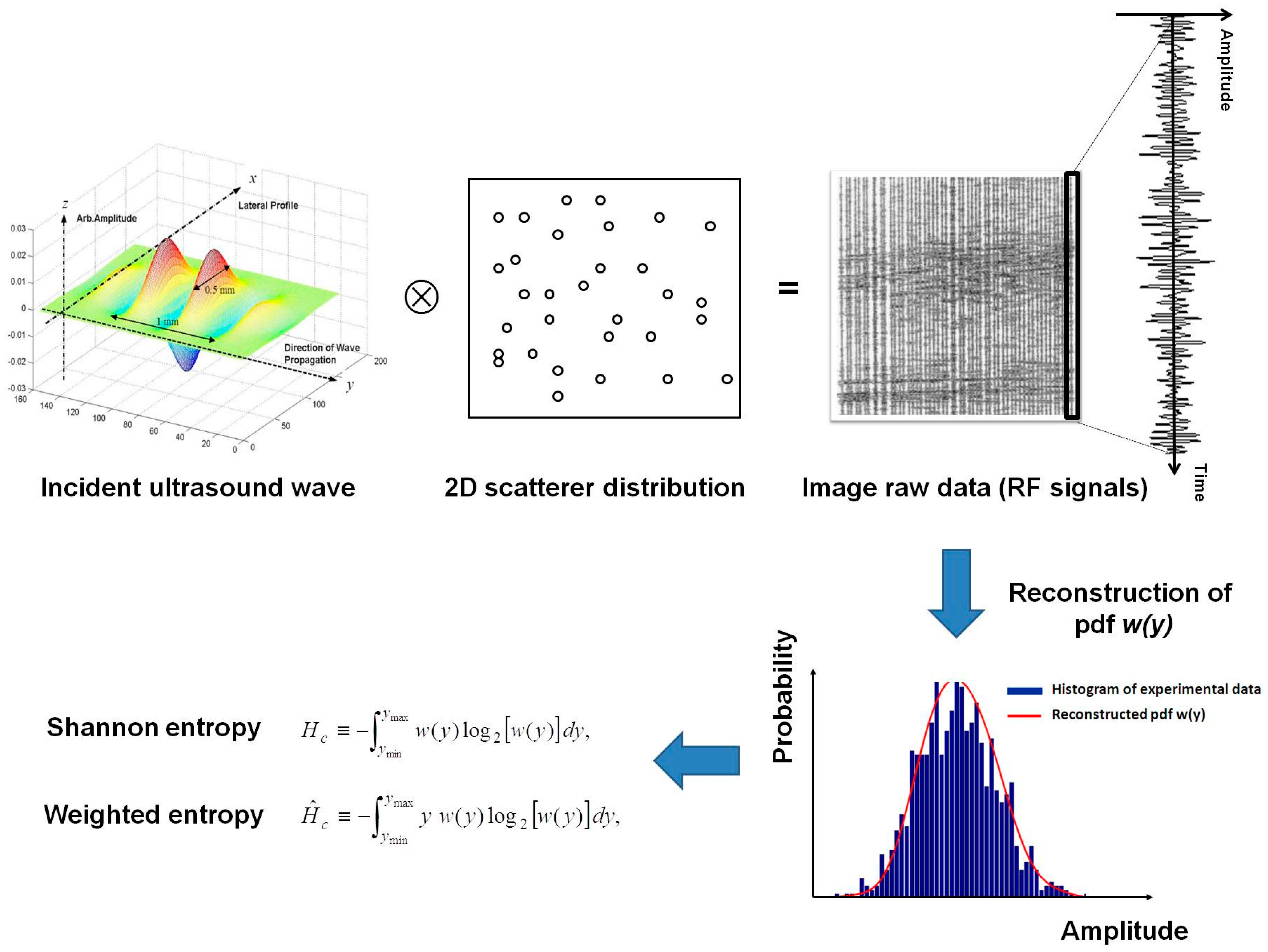
3.3. Selections of Parameters (Nco and Δy) for Estimating Weighted Entropy
3.4. Performance Evaluation of Weighted Entropy
4. Results
4.1. Effects of Nco and Δy on the Reconstruction of w(y)
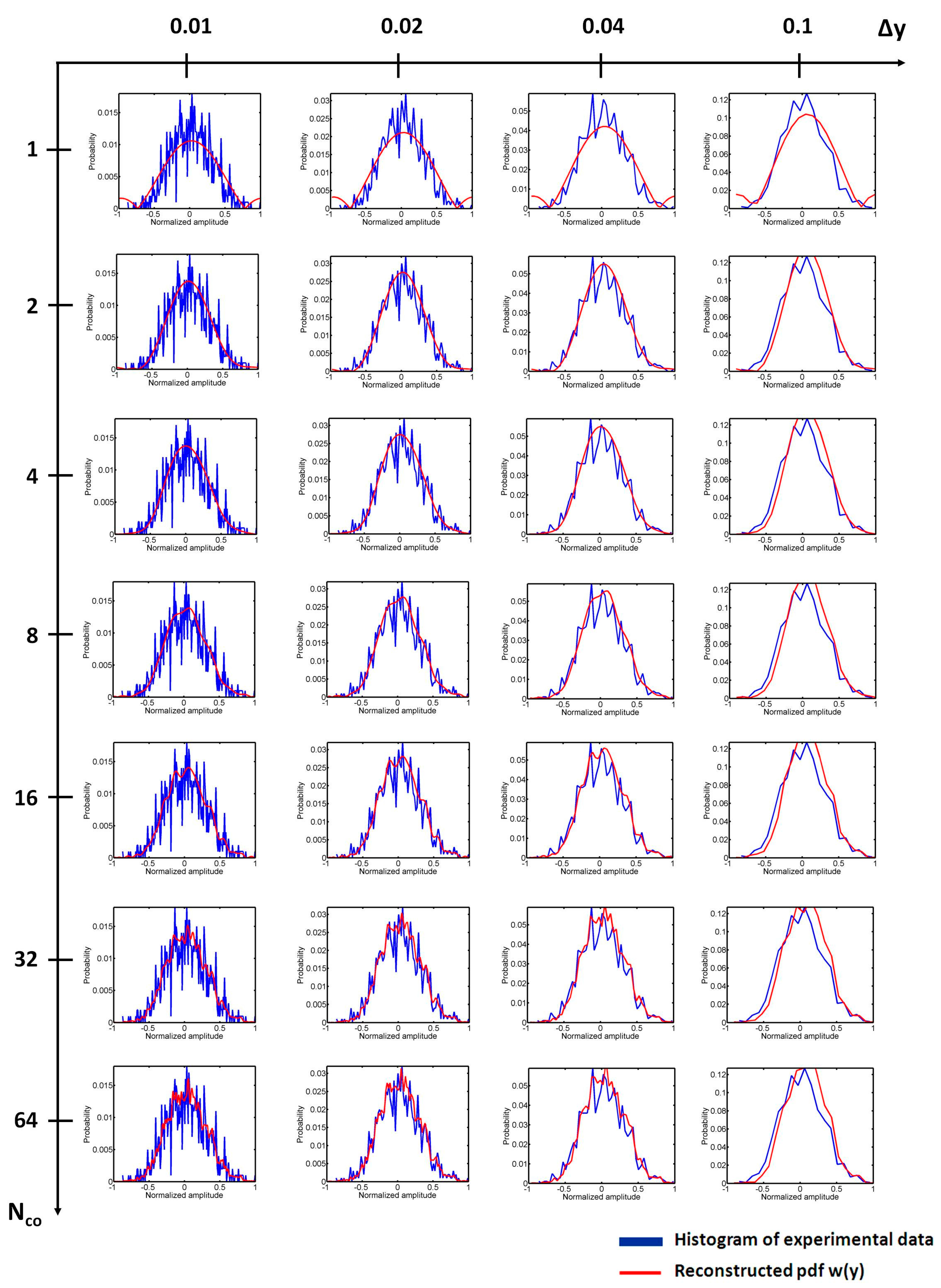
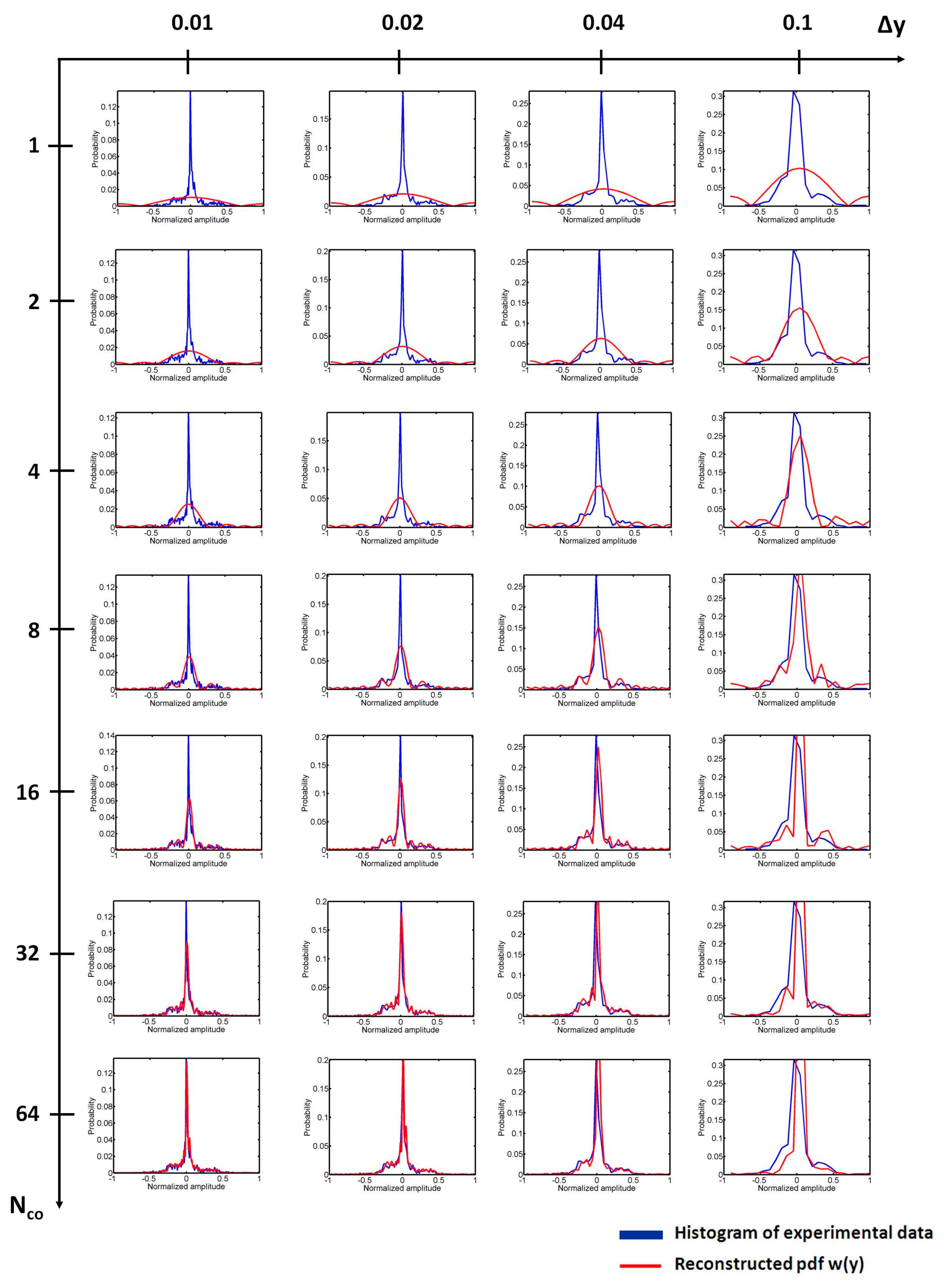
4.2. Determination of Nco


4.3. Determination of Δy
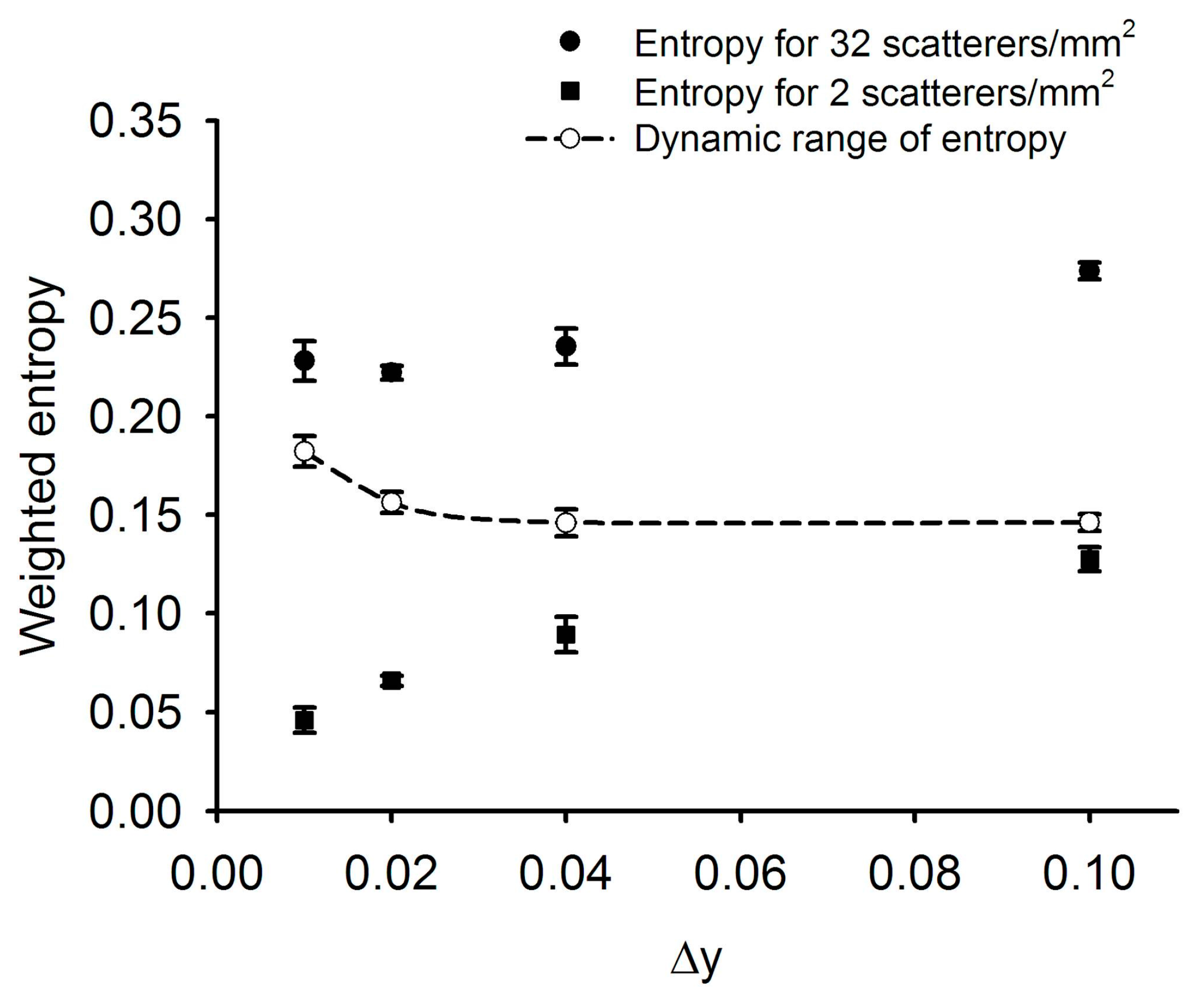
4.4. Weighted Entropy as a Function of Number Density of Scatterers
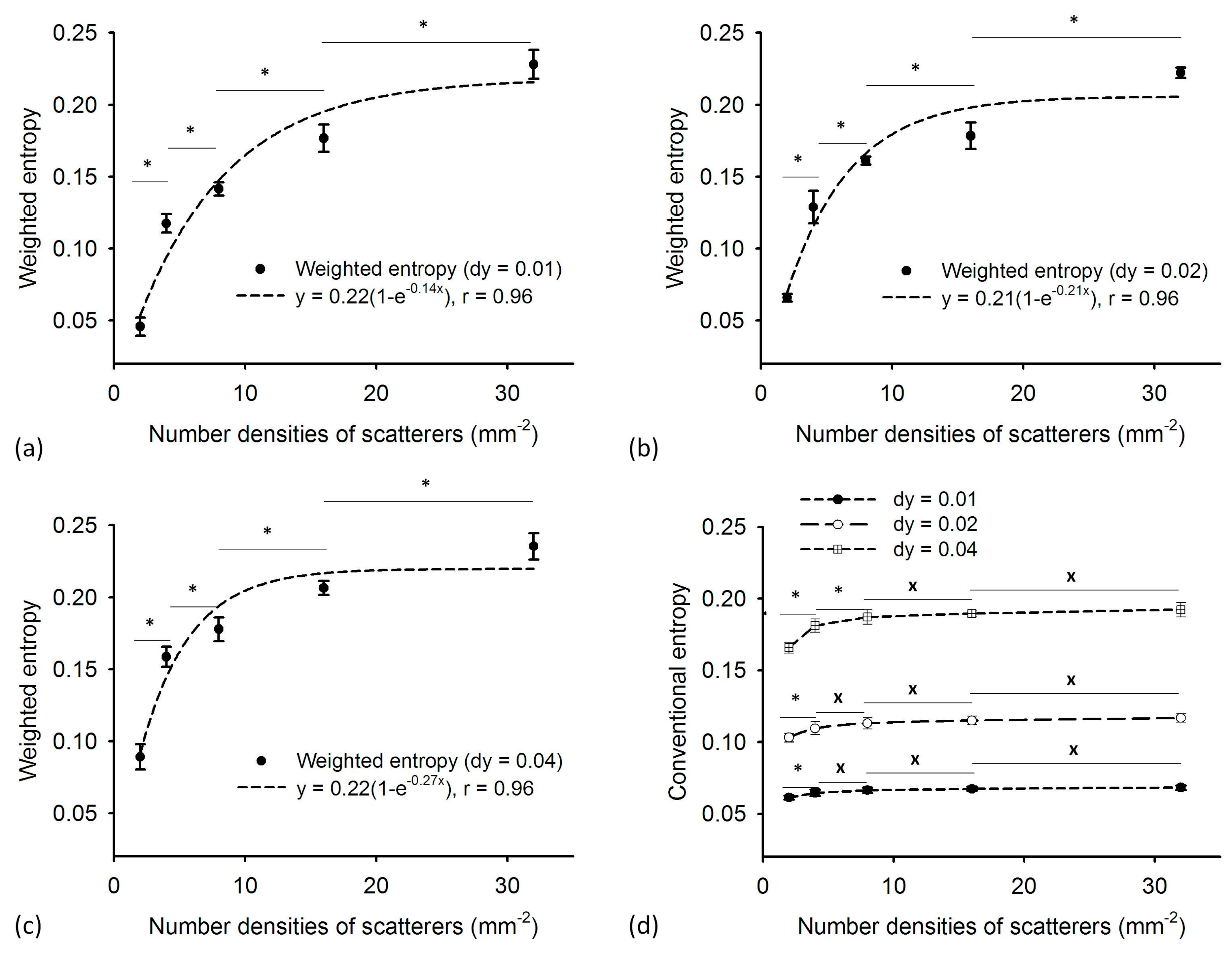
5. Discussion
5.1. Significance of This Study
5.2. Explanations on the Sensitivity Enhancement by Weighted Entropy
5.3. Relationship Between Distribution Parameter and Weighted Entropy
5.4. Biophysical Meanings of the Weighted Entropy and Future Work

6. Conclusions
Acknowledgements
Conflicts of Interest
References
- Destrempes, F.; Cloutier, G. A critical review and uniformized representation of statistical distributions modeling the ultrasound echo envelope. Ultrasound Med. Biol. 2010, 36, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.B. Speckle in ultrasound B-mode scans. IEEE Trans. Sonics Ultrasonics 1978, 25, 1–6. [Google Scholar] [CrossRef]
- Wagner, R.F.; Insana, M.F.; Brown, D.G. Statistical properties of radio-frequency and envelope detected signals with applications to medical ultrasound. J. Opt. Soc. Am. 1987, 4, 910–922. [Google Scholar] [CrossRef]
- Weng, L.; Reid, J.M.; Shankar, P.M.; Soetanto, K. Ultrasound speckle analysis based on the K distribution. J. Acoust. Soc. Am. 1991, 89, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Dutt, V.; Greenleaf, J.F. Ultrasound echo envelope analysis using a homodyned K distribution signal model. Ultrasonic Imaging 1994, 16, 265–287. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, E.; Tough, R.J.A. Generalized K distribution: A statistical model for weak scattering. J. Opt. Soc. Am. A 1987, 4, 1764–1772. [Google Scholar] [CrossRef]
- Liu, D.L.; Waag, R.C. Harmonic amplitude distribution in a wideband ultrasonic wavefront after propagation through human abdominal wall and breast specimens. J. Acoust. Soc. Am. 1997, 101, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Blacknell, D.; Tough, R.J.A. Parameter estimation for the K-distributed based on [z log (z)]. IEE Proc. Radar Sonar Navig. 2001, 148, 309–312. [Google Scholar] [CrossRef]
- Hruska, D.P.; Sanchez, J.; Oelze, M.L. Improved diagnostics through quantitative ultrasound imaging. In Proceedings of Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2009, Minneapolis, MN, USA, 3–6 September 2009; pp. 1956–1959.
- Destrempes, F.; Poree, J.; Cloutier, G. Estimation method of the homodyned K-distribution based on the mean intensity and two log-moments. SIAM J. Imaging Sci. 2013, 6, 1499–1530. [Google Scholar] [CrossRef] [PubMed]
- Holfman, H.C. Statistical Methods on Radio Wave Propagation; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Mamou, J.; Oelze, M.L. Quantitative Ultrasound in Soft Tissues; Springer: New York, NY, USA, 2013; Chapter 10; p. 220. [Google Scholar]
- Shankar, P.M. A general statistical model for ultrasonic backscattering from tissues. IEEE Trans. Ultrasonics Ferroelec. Freq. Control 2000, 47, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.M. A compound scattering pdf for the ultrasonic echo envelope and its relationship to K and Nakagami distributions. IEEE Trans. Ultrasonics Ferroelec. Freq. Control 2003, 50, 339–343. [Google Scholar] [CrossRef]
- Karmeshu; Agrawal, R. Study of ultrasonic echo envelope based on Nakagami-inverse Gaussian distribution. Ultrasound Med. Biol. 2006, 32, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R. Karmeshu Ultrasonic backscattering in tissue: Characterization through Nakagami-generalized inverse Gaussian distribution. Comput. Biol. Med. 2006, 37, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Smolikova, R.; Wachowiak, M.P.; Zurada, J.M. An information-theoretic approach to estimating ultrasound backscatter characteristics. Comput. Biol. Med. 2004, 34, 355–370. [Google Scholar] [CrossRef]
- Tsui, P.H.; Huang, C.C.; Sun, L.; Dailey, S.H.; Shung, K.K. Characterization of lamina propria and vocal muscle in human vocal fold tissue by ultrasound Nakagami imaging. Med. Phys. 2011, 38, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, C.C.; Shung, K.K.; Tsui, P.H.; Fang, J.; Ma, H.Y.; Wu, S.; Lin, C.C. Entropic imaging of cataract lens: An in vitro study. PLoS One 2014, 9, e96195. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, T.A.; Sperry, R.H.; Parker, K.J. Deviations from Rayleigh statistics in ultrasonic speckle. Ultrasonic Imaging 1988, 10, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Hughes, M.S. Analysis of ultrasonic waveforms using Shannon entropy. In Proceedings of IEEE 1992 Ultrasonics Symposium, Tucson, AZ, USA, 20–23 October 1992; pp. 1205–1209.
- Hughes, M.S. Analysis of digitized waveforms using Shannon entropy. J. Acoust. Soc. Am. 1993, 93, 892–906. [Google Scholar] [CrossRef]
- Hughes, M.S. Analysis of digitized waveforms using Shannon entropy. II. High speed algorithms based on Green’s functions. J. Acoust. Soc. Am. 1994, 95, 2582–2588. [Google Scholar] [CrossRef]
- Hughes, M.S.; McCarthy, J.E.; Marsh, J.N.; Arbeit, J.M.; Neumann, R.G.; Fuhrhop, R.W.; Wallace, K.D.; Znidersic, D.R.; Maurizi, B.N.; Baldwin, S.L.; et al. Properties of an entropy-based signal receiver with an application to ultrasonic molecular imaging. J. Acoust. Soc. Am. 2007, 121, 3542–3557. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.S.; McCarthy, J.E.; Marsh, J.N.; Wickline, S.A. Joint entropy of continuously differentiable ultrasonic waveforms. J. Acoust. Soc. Am. 2013, 133, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Nawrockia, D.N.; Harding, W.H. State-value weighted entropy as a measure of investment risk. Appl. Eco. 1986, 18, 411–419. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Peng, N.; Ling, J. Weighted information entropy: A method for estimating the complex degree of infrared images’ backgrounds. In Image Analysis and Recognition; Kamel, M., Campilho, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume: 3656, pp. 215–222. [Google Scholar]
- Khan, J.F.; Bhuiyan, S.M. Weighted entropy for segmentation evaluation. Opt. Laser Technol. 2014, 57, 236–242. [Google Scholar] [CrossRef]
- Guiasu, S. Weighted entropy. Rep. Math. Phys. 1971, 2, 165–179. [Google Scholar] [CrossRef]
- Lai, W.K.; Khan, I.M.; Poh, G.S. Weighted entropy-based measure for image segmentation. Procedia Eng. 2012, 41, 1261–1267. [Google Scholar] [CrossRef]
- Yeung, R.W. A First Course in Information Theory; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Chapter 1; p. 2. [Google Scholar]
- Tsui, P.H.; Chang, C.C.; Ho, M.C.; Lee, Y.H.; Chen, Y.S.; Chang, C.C.; Huang, N.E.; Wu, Z.H.; Chang, K.J. Use of Nakagami statistics and empirical mode decomposition for ultrasound tissue characterization by a nonfocused transducer. Ultrasound Med. Biol. 2009, 35, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Shung, K.K.; Thieme, G.A. Ultrasonic Scattering in Biological Tissues; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Jensen, J.A. Simulation of advanced ultrasound systems using Field II. In Proceedings of IEEE International Symposium on Biomedical Imaging: Nano to Macro, 2004, Prague, Czech Republic, 15–18 April 2004; pp. 636–639.
- Jensen, J.A.; Svendsen, N.B. Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers. IEEE Trans. Ultrasonics Ferroelec. Freq. Control 1992, 39, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, I.; Bertrand, M.; Cloutier, G. A system-based approach to modeling the ultrasound signal backscattered by red blood cells. Biophys. J. 1999, 77, 2387–2399. [Google Scholar] [CrossRef]
- Azhari, H. Basics of Biomedical Ultrasound for Engineers; Wiley: New York, NY, USA, 2010; Chapter 9; p. 200. [Google Scholar]
- Tsui, P.H.; Chang, C.C. Imaging local scatterer concentrations by the Nakagami statistical model. Ultrasound Med. Biol. 2007, 33, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.H. Minimum requirement of artificial noise level for using noise-assisted correlation algorithm to suppress artifacts in ultrasonic Nakagami images. Ultrasonic Imaging 2012, 34, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.H.; Ma, H.Y.; Zhou, Z.; Ho, M.C.; Lee, Y.H. Window-modulated compounding Nakagami imaging for ultrasound tissue characterization. Ultrasonics 2014, 54, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Zagzebski, J.A.; Chen, J.F.; Dong, F.; Wilson, T. Intervening attenuation affects first-order statistical properties of ultrasound echo signals. IEEE Trans. Ultrasonics Ferroelec. Freq. Control 1999, 46, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, S.; Wang, C.Y.; Ma, H.Y.; Lin, C.C.; Tsui, P.H. Monitoring radiofrequency ablation using real-time ultrasound Nakagami imaging combined with frequency and temporal compounding techniques. PLoS One 2015, 10, e0118030. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, C.K.; Peng, J.Y.; Hsu, C.H.; Jeng, Y.M.; Lee, Y.H.; Lin, J.J.; Tsui, P.H. Changes in backscattered ultrasonic envelope statistics as a function of thrombi age: An in vitro study. Ultrasound Med. Biol. 2015, 41, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.H.; Yeh, C.K.; Chang, C.C.; Chen, W.S. Performance evaluation of ultrasonic Nakagami image in tissue characterization. Ultrasonic Imaging 2008, 30, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.H.; Tsai, Y.W. Artifact reduction of ultrasound Nakagami imaging by combining multifocus image reconstruction and the noise-assisted correlation algorithm. Ultrasonic Imaging 2015, 37, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Duitama, J.; Chayer, B.; Han, A.; Garcia, D.; Oelze, M.L.; Cloutier, G. Experimental application of ultrafast imaging to spectral tissue characterization. Ultrasound Med. Biol. 2015, 41, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsui, P.-H. Ultrasound Detection of Scatterer Concentration by Weighted Entropy. Entropy 2015, 17, 6598-6616. https://doi.org/10.3390/e17106598
Tsui P-H. Ultrasound Detection of Scatterer Concentration by Weighted Entropy. Entropy. 2015; 17(10):6598-6616. https://doi.org/10.3390/e17106598
Chicago/Turabian StyleTsui, Po-Hsiang. 2015. "Ultrasound Detection of Scatterer Concentration by Weighted Entropy" Entropy 17, no. 10: 6598-6616. https://doi.org/10.3390/e17106598




