2. Illuminating Water Creates Coherence
Del Giudice has literally brought light into the quantum physics of water, thereby also illuminating life. Standard quantum theory does not predict quantum coherence for liquid water, because it ignores both quantum fluctuations and the interaction between matter and light, the electromagnetic vacuum field. These are only taken into account in quantum electrodynamics field theory. However, conventional quantum electrodynamics field theory applies only to gases.
Working with his close associate, Giuliano Preparata (1942–2000), Del Giudice and other colleagues, especially Giuseppe Vitiello, Antonella De Ninno and Alberto Tedeschi, extended conventional quantum electrodynamics theory to the condensed phase of liquids. They showed that interaction between the vacuum electromagnetic field and water induces coherent excitations that lead to the formation of large, stable coherent domains (CDs) about 100 nm in diameter, resulting in the condensation to the liquid phase at some critical density. These CDs are present in liquid water at ordinary temperature and pressure and may be responsible for all of the special properties of water, including life itself [
6–
10].
Each CD of water is effectively a resonating cavity produced by the electromagnetic field that ends up trapping the field, because the photon acquires an imaginary mass, so the frequency of the CD electromagnetic field becomes much smaller than the frequency of the free field with the same wavelength.
Under ambient conditions, water is an approximately equal mixture of CDs surrounded by incoherent regions (more accurately, the water molecules are dancing between the CD and non-CD configurations, and both the CD and non-CD molecules are interchangeable), This picture is reflected in the many observations supporting a two-state model of liquid water, a dense state and a less dense state co-existing simultaneously. The less dense state with tetrahedral-directed, fully hydrogen-bonded water molecules is the excited state and, hence, corresponds to water in the CD, while the non-CD water molecules represent the dense state in which the hydrogen bonding is not so regular.
3. Coherent Water at Interfaces Makes Life Possible
According to Del Giudice and colleagues, the water molecules in the CD are oscillating between the ground state and an excited state of 12.06 eV, just below the first ionization potential of 12.56 eV, and, therefore, contain close to a million almost-free electrons; the proportion of excited state molecules within the CD is estimated to be 0.13 (in a 2012 publication [
11], these values are re-estimated from empirical data to be 12.07 eV and 12.62 eV, respectively, but the main argument remains unchanged). That means CDs are most likely negatively charged at the periphery close to or at the surface of the sphere (at the same time, positively-charged protons are probably present just outside the coherent domain). The surface of the CD is thus “a redox pile”, where the almost-free electrons can be readily donated to electron acceptors. Excited coherent water is the basis of the oxidation-reduction energy metabolism that powers all living processes; it is both the chemistry and the electricity of life, and as we shall see, it orchestrates all of the necessary chemical reactions [
4].
The abundant life on Earth, including the human species, depends ultimately on photosynthesis in green plants, algae and cyanobacteria. In the process, sunlight is trapped by chlorophyll to split water into hydrogen, electrons and oxygen (
Equation (1)), giving life access to an abundant energy source and, perhaps more importantly, liberating oxygen for the evolution of air-breathing organisms that filled Earth with the teaming millions of species.
The hydrogen ion (protons) and electrons go to reduce (or fix) carbon dioxide into the carbohydrates and biomass of photosynthetic organisms, which feed herbivores, and down the food web, the vast majority of animal species. The air-breathers break down carbohydrates by oxidizing them (with oxygen) in the mitochondria of cells to obtain energy for growth and reproduction, regenerating carbon dioxide and water. This completes the living dynamo of photosynthesis and respiration that turns inanimate substances into living organisms.
It takes 12.56 eV to split water, an energetic photon of a wavelength of 98.7 nm in the soft X-ray region that would destroy life. However, photosynthesis depends mainly on red light (~680 nm, 1.8 eV) and, to some extent, blue light in the visible spectrum. Therefore, how is that possible? It is possible on account of the excited water in the coherent domains.
More than 50 years ago, Nobel Laureate and father of biochemistry, Albert Szent-Györgyi, had already suggested that water at interfaces is the key to life and proposed that water at interfaces, such as membranes, is in the excited state, requiring considerably less energy to split than water in the ground state [
12]. A sign of the excited water is that a voltage should appear at the boundary between interfacial water and bulk water, which was indeed observed not long after Szent-Györgyi made his prediction (see [
9]). Most, if not all, water in living organisms is interfacial water, as it is almost never more than a small fraction of a micron away from surfaces, such as membranes or macromolecules. Water at the interface is a coherent domain generated by the interaction of the electromagnetic field and water and stabilized by the interface, as Del Giudice and colleagues suggested [
10].
Unexpected support for the theory came from a field study on aerosols generated at five water falls in the Austrian Alps carried out by Pierre Madl at Salzburg University in Austria with Del Giudice and other collaborators around the world. The aerosols showed a bimodal size distribution with small clusters a few nanometres in diameter consisting of a few hundred water molecules and larger aggregates about 100–200 nm in diameter with millions of water molecules [
13]. Whereas the small clusters disappear very rapidly with distance from the falls, the larger aggregates are able to propagate for hundreds of metres. The aggregates detected, both large and small, are electrically charged with negative charge predominating for 85% of aggregates. The existence of the surface electrical charge and unusual size distribution of the aggregates are both predictions of the quantum electrodynamics theory of water; although, the precise details have yet to be worked out.
4. Understanding the Quantum Physics of EZ Water
Gerald Pollack’s laboratory at University of Washington Seattle in the United States has provided a most vivid demonstration of interfacial water, and the fascinating story is told in Pollack’s excellent book,
The Fourth Phase of Water [
14]. A hydrophilic gel in a suspension of microspheres just visible to the eye created interfacial water that extends hundreds of microns from the surface of the gel, excluding the microspheres, as well as other solutes, such as proteins and dyes, and, hence, is referred to as an “exclusion zone” (EZ) (see also [
15]).
EZ water appears to be 10-times more viscous than bulk water and its refractive index and density about 10% higher. It has many other distinctive properties indicative of structure, including a characteristic peak of light absorption at 270 nm. Del Giudice and colleagues suggested that EZ water is a gigantic coherent domain stabilized on the surface of the attractive gel, though the higher density of EZ water rules out the tetrahedral-directed ice-like hydrogen bonds proposed for water in the coherent domains in free solution. Pollack has indeed suggested an entirely different structure for EZ water [
14].
In line with the formation of coherent domains in quantum electrodynamics theory, there is a negative electric potential associated with EZ water, which enables it to act as a battery, as demonstrated in the Pollack lab (see [
16]). However, the surface charge of the EZ water depends on the surface charge of the hydrophilic gel on which it is formed. While a negatively charged gel resulted in a negative potential of the EZ with respect to the bulk water, with a low pH zone immediately next to it, a positively charged hydrophilic gel gives a positive potential at the surface of the EZ with a high pH zone adjoining [
17]. Further, the magnitude of the potential difference also seems to be determined by the hydrophilic gel, independently of the thickness of the EZ.
In line with quantum electrodynamics theory, EZ water appears to be formed by the interaction of light with the water; the most effective light being far infrared/microwave ~3000 nm. The gap between the ionizing potential of 12.56 eV and the excited level of 12.06 eV predicted by Del Giudice and colleagues is 0.5 eV, equivalent to 2479.7 nm, not too different from ~3000 nm (equivalent to 0.413 eV); although the 3000-nm absorption may have more to do with the rearrangement of CDs into EZ water rather than charge separation. This infrared absorption maximum in wave number, ~3333 cm
−1, is close to the 3350 cm
−1 identified as the spectral signature of four-coordinated sites in water clusters [
18], as distinct from that of ice at 3220 cm
−1.
The peak absorption of EZ water at 270 nm (4.59 eV) is much longer than the 102.8 nm equivalent of 12.06 eV predicted from quantum electrodynamics theory. It should be noted that the value of 12.06 or 12.07 eV is selected as the most likely excitation level that fit with phenomena (including the fact that photosynthesis requires energetic photons of only 1.8 eV) and applies to water in the gas phase [
9,
14]. Quantum chemical calculations of neutral and charged (H
2O)
n water clusters modelling fragments of a real hydrogen bond network of water and dynamic simulations of the clusters, either upon electron removal from a stable neutral cluster or upon the excitation of various cluster vibrations, enabled separate stages of structure reorganization to be distinguished [
19]. The energy necessary for the ionization of superficial water layers at irradiation was estimated from the intermediate ionization potentials of water clusters to be 9.5 eV (130.5 nm). The experimental value obtained on a rotating quartz disc was 9.3 eV. That is still far higher than the 4.59 eV (270 nm) for the formation of EZ water. There are indeed lower levels of excitation for water. We shall return to this important issue later.
5. Understanding Structured Water
EZ water is structured water, according to spectroscopic and NMR studies, as well as birefringence under polarized light microscopy [
14]. That does not mean structure is absent in bulk water, as the extended hydrogen-bonded networks and coherent domains both predict structure in bulk water.
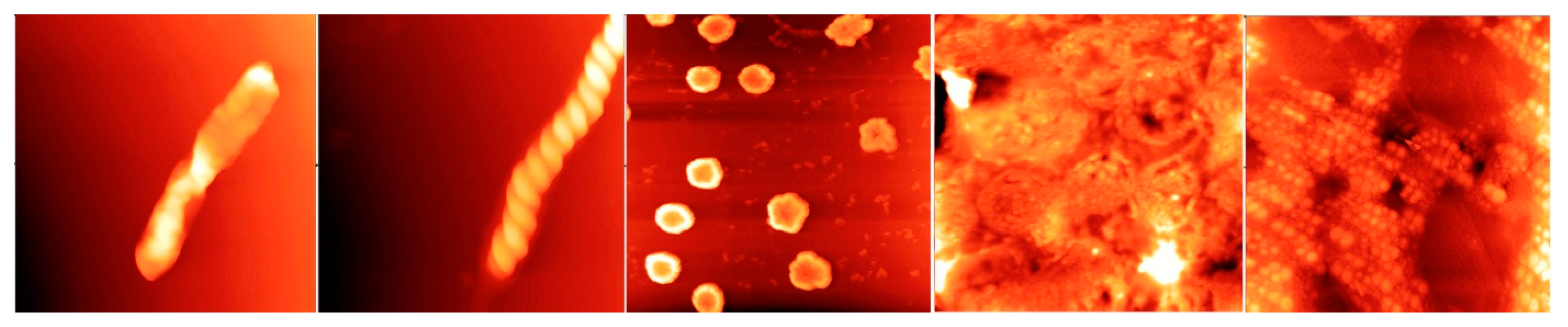
Indeed, stable water clusters tens of nanometres to millimetres in dimension can be isolated from bulk water and imaged under transmission electron microscope (TEM) and atomic force microscope (AFM) [
20–
22], as first demonstrated in Shui-Yin Lo’s laboratory at the Institute of Quantum Medicine, Pasadena, California, in the United States (
Figure 1). The clusters consisting of millions to billions of water molecules appear in a wide variety of flexible structures that could be deformed by the tips of the atomic force microscope probe if scanned in the contact mode. Otherwise, they remained stable for weeks and months at room temperature and pressure. They have all the characteristics of “soft matter”—liquids, liquid crystals, colloids, polymers, gels and foams—that form mesoscopic structures much larger than the molecules themselves, but small compared with the bulk material.
The structures were typically prepared by serially diluting a solution of pure sodium chloride with vigorous shaking (succussion) in ultrapure distilled de-ionised water in a low-dust room until it is about 10−6 M or 10−7 M, then placing drops to dry on a clean glass slide or some other substrate. Close-up, the diverse structures appear to be composed of spherical “balls” 50–100 nm in diameter. Under the electron microscope, they are electron dense at the surface.
The effect of dilution, as explained by Lo and colleagues [
20–
22], was to enable dipole interactions between water molecules to dominate over ionic interactions and may be crucial in forming the supramolecular structures observed.
Similar structures have been produced since by several research groups (reviewed in [
23]). Significantly, they contain strong electric fields, with reported absorption maxima variously at 195 nm, 230.6 nm and 276.7 nm, perhaps not too dissimilar to the 270 nm of EZ water.
Vittorio Elia at University of Naples and colleagues successfully imaged large water clusters in water repeatedly brought into contact with Nafion [
24] or repeatedly filtered [
25]. The “Nafionated water”, which they equated with EZ water, showed an increase in electrical conductivity by up to two orders of magnitude. At the same time, there was a drop in pH from ~6 to 3, representing a three orders of magnitude increase in proton concentration. The increase in conductivity was attributed to proton conduction [
26], suggesting that protons are present in the clusters. In other words, their clusters include not only EZ water, but also water immediately next to it, which has been shown to be enriched in protons in the case of Nafion [
14,
16]. Lyophilization of 20 mL of the Nafionated water gave 1 to 2 mg of residue, and AFM confirmed the presence of micron sized structures that look superficially similar to the large water clusters identified in the Lo laboratory [
20].
However, repeatedly filtered water, while showing the same increase in conductivity, was accompanied by an increase in pH compared with the starting distilled deionized water [
25]. This suggests that the nature of the charged groups on the conditioning substrate—silicate, acetate and nitrate groups in the sintered glass, cellulose acetate and nitrocellulose filters
versus sulphonate groups in Nafion—and the nature of physical contact will both affect the net charge of the conditioned water, the same as for the EZ water [
17]. What the treated waters share in common is the separation or near-separation of charges due to coherent excitation of water as predicted by quantum electrodynamics theory [
6–
11].
Independently, work carried out in the laboratory of Alexander Konovalov at Russian Academy of Sciences, Kazan Tatarstan, shows that highly serially diluted solutions, regardless of whether they involve organic salts, amphiphilic or hydrophobic compounds, spontaneously form clusters 100 to 300 nm in size with a surface potential −2 to −20 mV (reviewed in [
27]). The team characterized the nanostructures in solution in terms of conductivity, size, surface tension and surface potential at a wide range of concentrations, but not direct imaging of the nanostructures. A significant finding is that the nanostructures fail to form when the diluted solutions are placed in a container shielded by permalloy to exclude electromagnetic fields [
28]. This suggests that interaction with the ambient electromagnetic field is essential for the formation of supramolecular water clusters, as predicted from quantum electrodynamics field theory and demonstrated in Pollack’s EZ above. This important observation merits further investigation.
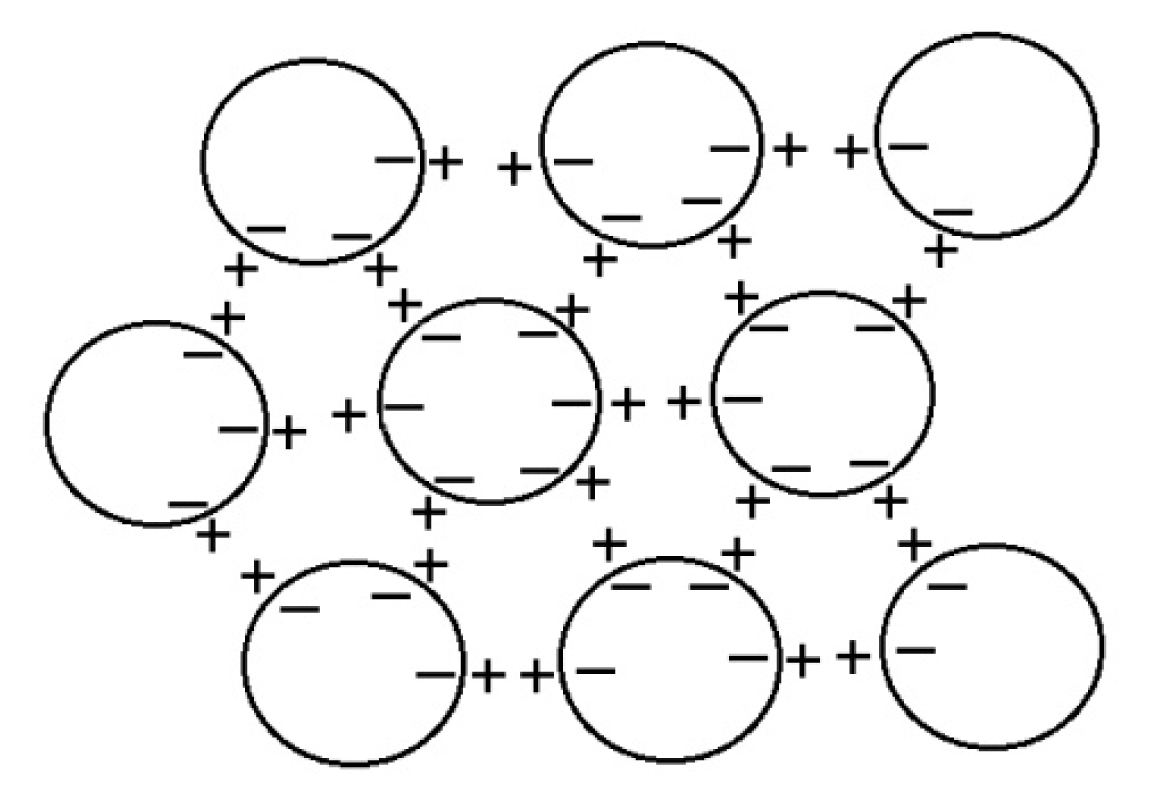
5.1. Symmetrical Spherical Dipoles from Coherent Domains
Lo and colleagues [
20] suggested that the supramolecular structures are made of small unit “balls” ~100 nm that are “dipoles”. However, these are not ordinary dipoles. Instead, I proposed that they may be coherent domains predicted in quantum electrodynamics theory [
23]. They are about the right size, each having close to a million almost-free electrons at the periphery, along with positively charged protons as in EZ water [
14–
17] and Nafionated water [
24,
26]. Consequently, these spherical CDs can mimic dipole interactions through negative charges on their periphery attracting positive charges just outside to form a three-dimensional potentially perfectly symmetrical giant electret (dipole). Such an electret structure will give an electric field measured in any direction as found by Lo [
22] and also by Roberto Germano at Promete Srl San Giorgio a Cremano, working with Emilio and others in Nafionated water [
29]. This formation of an electret also explains the enormous increase in conductivity in Nafionated [
24,
26] and repeatedly filtered water [
25]. When isolated by drying on a substrate, the symmetry is broken, and the clusters adopt a variety of “snowflake”-like structures with six-fold symmetry, which arise from the close packing of spheres in my model (
Figure 2).
Another prediction from quantum electrodynamics field theory [
8,
10] is consistent with the structure of the clusters proposed here and may account for their apparent stability. The coherent oscillations maintained by the electromagnetic field trapped within the CDs can occur not just between the coherent ground state and excited state of the electrons of the water molecule, but also between two rotational levels, which produce correlations as large as several hundred microns, giving rise to a common dipole orientation, but a net zero polarization field (on account of its symmetry), unless and until the rotation symmetry is broken. The combination of the coherent oscillations and rotations, therefore, produces phase-locked coherent interactions among the CDs, resulting in stable supramolecular clusters with the electret structure depicted in
Figure 2. The diagram should be read quantum mechanically, with delocalized clouds of oscillating positive and negative charges between coherently rotating domains.
6. Quantum Delocalization and Superconducting Protons
The separation of positive and negative charges in the quantum electrodynamics theory of water is important for intercommunication, especially in the form of protons. I have considered proton conduction in living organisms since the first edition of
Rainbow Worm [
3] published in 1993. Later, I proposed with David Knight that water aligned along collagen fibres in connective tissues could be the anatomical basis of the acupuncture meridians [
30], enabling cells and tissues to intercommunicate via proton currents. I also suggested that water confined in nanospaces, such as carbon nanotubes and collagen fibres, could be proton superconducting [
31,
32]. There is now good evidence in support of that hypothesis [
33], which I shall briefly describe.
It is generally accepted that the key to water’s remarkable properties is the hydrogen bond interconnecting the water molecules. Linus Pauling was the first to suggest that the hydrogen bond is partly covalent in 1935 [
34]. In 1999, Inelastic X-ray scattering on a carefully prepared slab of ice yielded results that supported Pauling’s proposal [
35]; the data fit a quantum mechanical model rather than a classical electrostatic model for the hydrogen bond.
In 2002, researchers at the FOM (Foundation for Fundamental Research on Matter) Institute of Atomic and Molecular Physics in the Netherlands used ultrafast femto-second pulses of infrared light to excite and probe the O-H covalent bond in liquid water at room temperature [
36]. Again, the results support a quantum mechanical model in which the excited proton could be found simultaneously at a distance of the equilibrium O-H bond from both its neighbour oxygen on either side and at a much reduced energy for the excited (v = 2) state than if the hydrogen bond did not exist.
The energy of excitation to the v = 2 delocalized state, estimated at 0.806 eV (6500 cm
−1 or 1538.5 nm) is less than 20% of the O-H bond energy of 4.8 eV (38,750 cm
−1, 258.1 nm), estimated from the O-H stretch vibration frequency. The energy required for excitation to the delocalized state is very much smaller than the 12.06 eV for excitation to coherent domains [
6], and the O-H bond energy at 4.8 eV is also much smaller than the value of 12.56 eV required for ionization. I believe these results are telling us something very significant: it is much easier for H
+ to dissociate from the water molecule than for the water molecule to lose an electron. The energy of 4.8 eV is equivalent to light at 258.1 nm, which is close to the absorption peak of EZ water and consistent with the loss of H
+ from EZ, the H
+ accumulating at the interface of EZ with bulk water. Consequently, the activity of protons derived from water may be much more important for living systems than electrons and in the form of proton currents. Delocalization of protons, by absorption of ambient photons at 1538.5 nm, increases the probability of proton transfer,
i.e., proton conduction could take place much more readily and rapidly. It would be easy to check if the predicted 1538.5 nm does promote proton conduction; if so, it could have important clinical applications.
Water confined in nanospaces is even more remarkable, and that applies to most biological water. It adopts new quantum states, resulting in proton conduction rates orders of magnitude higher than in bulk water.
7. New Quantum States of Water in Nanospaces and Superconducting Protons
Research led by George Reiter at the University of Houston Texas uses deep neutron inelastic scattering to measure the momentum distribution of protons in water confined to nanospaces. The momentum of the proton is mainly determined by the wave-function of the proton’s ground-state (least energetic state).
The team investigated carbon nanotubes, glass sponge and Nafion membranes and found similar results [
37,
38]. The confined water adopts a variety of new quantum states distinct from bulk water and highly dependent on the precise dimensions of the nanospace. In other words, water adopts a single quantum state, wherever it is, which is quite remarkable in itself (I invite you to think about the ocean, when it is clear and calm and when raging with tsunami (see [
39])). Water confined in space dimensions of 2 nm or smaller has protons that are coherently delocalized in two momentum states.
In xerogel, a glass sponge with Si-OH (silanol) groups lining the pores that can hydrogen bond with water, the proton momentum distribution of water in 24 Å pores at room temperature is confined in a double-well potential. For larger pores of 82 Å, the average momentum distribution was close to that of bulk water, though still quite distinct [
37].
The perfluorosulphonic acid membranes, Nafion 1120 and Dow 858, are polymers consisting of a hydrophobic poly(tetrafluoroethylene) backbone and randomly distributed side chains of perfluoroether terminating with sulphonic acids. When hydrated, nanophase separation occurs in which water is confined in domains of a few nanometres in diameter surrounded by hydrophobic regions. The sulphonic acid group (-SO
3H) donates its proton to water when there is sufficient water in the pores, making them very good proton conductors. The momentum distributions at room temperature for water in the two membranes are dramatically different from water in the bulk: the kinetic energy is higher in Nafion by 107 meV/proton and in Dow 858 by 124 meV/proton. They are qualitatively different quantum states from bulk water. At a concentration of 14 H
2O/SO
3H for both membranes, Dow 858 has a significantly higher conductivity by 70% than Nafion, consistent with a higher degree of proton delocalization [
38].
In a later publication, X-ray Compton scattering was used to probe the electronic ground state of nanospace-confined water [
40]. They found that the difference in “bond disorder” between water confined in Nafion and bulk water is 17-times larger than that between bulk water just above the freezing point and just below the boiling point. That is not surprising given that the proton is coherently distributed in double wells separated by ~0.3 Å. The kinetic energy has gone up because each of these wells is more tightly binding the proton than the covalent bond of the isolated water molecule. The kinetic energy (measured by deep inelastic neutron scattering) is 245 meV and 268 meV for Nafion and Dow, respectively, compared with 148 meV for bulk water at room temperature. The change in kinetic energy in going from 5 °C to 95 °C for bulk water is only 0.5 meV.
Further support for the new quantum states of nanospace-confined water comes from excited state proton transfer measurements of a fluorescent probe molecule, 8-hydroxypyrene-1,3,5-trisulphonate (HPTS). HPTS tends to stay in the middle of the water-filled regions in Nafion. When excited by a laser pulse, the proton in the OH group of HPTS is ionized and rapidly transferred to the surrounding solvent [
41]. The recombination time depends on the transport processes affecting the free proton. Diffusion in bulk water showed
t−1.5 dependence. In Nafion, the rate observed is much slower at
t−0.8, because it requires the redistribution of electrons through the hydrogen-bonded network. Similar results were obtained in reverse micelles, droplets of aqueous solution enclosed in a lipid membrane and dispersed in an organic solvent. Both reverse micelles and the water-filled nanospaces in Nafion are similar to the nanospaces inside the cell. Additionally, these findings will certainly have implications for biochemical reactions in the cell (see later).
Hydrated Nafion consists of long parallel, but otherwise randomly-packed water channels surrounded by partially hydrophilic side branches, forming inverse-micelle cylinders. At 20% by volume of water, the water channels have diameters between 1.8 and 3.5 nm with an average of 2.4 nm [
42].
Nafion films have a proton conductivity of about 0.1 S/cm (S, Sieman = 1 Ampere/Volt), among the highest in proton exchange membranes (PEMs). For a comparison, the electrical conductivity of copper is 596,000 S/cm and silicon, a semiconductor, 0.156 S/cm [
43]. However, the conductivity of a single high purity Nafion nanofibre 400 nm in diameter made by electrospinning reached 1.5 S/cm, an order of magnitude greater, as demonstrated by Yossef A Elabd at Drexel University Philadelphia in PA, USA, and his colleagues [
44]. This is due to the alignment of interconnected ionic aggregates along the fibre axis direction, as evidenced by X-ray scattering.
8. Proton Transport through Carbon Nanotubes
Very fast proton transport was reported in carbon nanotubes, ~40-times the rate in bulk water in molecular dynamic simulations [
45]. More recent
ab initio (starting from first principles) path-integral (quantum approach) molecular dynamics simulations showed no energetic barrier to proton transfer in every case when quantum delocalization is taken into account. The main difference between bulk liquid water and water confined in a carbon nanotube is a favourable pre-alignment of water molecules in the latter case. Configurations where the excess proton is quantum delocalized over several adjacent water molecules along with continuous interconversion between different hydration states reveal that, as in bulk water, the hydrated proton under confinement is best described as a fluxional defect, rather than any individual hydration state of the excess proton propagated classically along the water chain [
46].
Wonjoon Choi at Massachusetts Institute of Technology Cambridge in the USA led an investigation into the diameter-dependence of proton transport through the interior of isolated, specially fabricated single-walled carbon nanotubes 1 mm long, and demonstrated a surprising five-fold enhancement of stochastic proton transport rates at a diameter of approximately 1.6 nm, dropping off sharply to either side of the peak [
47]. The diameter of 1.6 nm is near the transition between water behaving rather as it would in an ordinary capillary tube at diameters >1.6 nm and water behaving just the opposite way at diameters <1.4 nm in a temperature-diameter phase diagram [
48]. At a diameter of ~1.5 nm, double- or triple-walled water nanotube structures have been proposed. Whether these structures are responsible for the sharp increase in proton conduction at 1.6 nm is not yet known.
Not only does nanospace-confined water show high temperature superconductivity, it also exhibits superfluidity, with a flow rate enhancement of 50- to 900-fold, depending on diameter, compared with that predicted from conventional fluid-flow theory [
52]. This has large implications for water transport into cells and electrolyte balance crucial for the health of cells and organisms.
These results of carbon nanotube-confined water are most relevant to water associated with collagen fibres.
9. Collagen Fibres and Acupuncture Meridians
Evidence dating back to the 1970s indicates that collagen does conduct protons. G.H. Bardelmeyer in The Netherlands [
50] found that the electrical conductivity of the bovine Achilles tendon is fully determined by the water of hydration, and the electric current is primarily carried by protons at water contents up to 45% and by small ions at water contents beyond 65%. Between water contents of 8.5% and 126%, conductivity went up eight orders of magnitude. He estimated that pure water’s dissociation constant is 10
−5 that of adsorbed water;
i.e., adsorbed water is five orders of magnitude more likely to let go of protons. Similarly, Naoki Sasaki in Japan found that the conductivity of collagen increased markedly with water absorbed, at an exponent of 5.1–5.4, between a water content of 0.1 to 0.3 g/g [
51]. These results make sense in light of the recent observations on proton superconduction in nanospace confined water; and new measurements on hydrated collagen fibres need to be done.
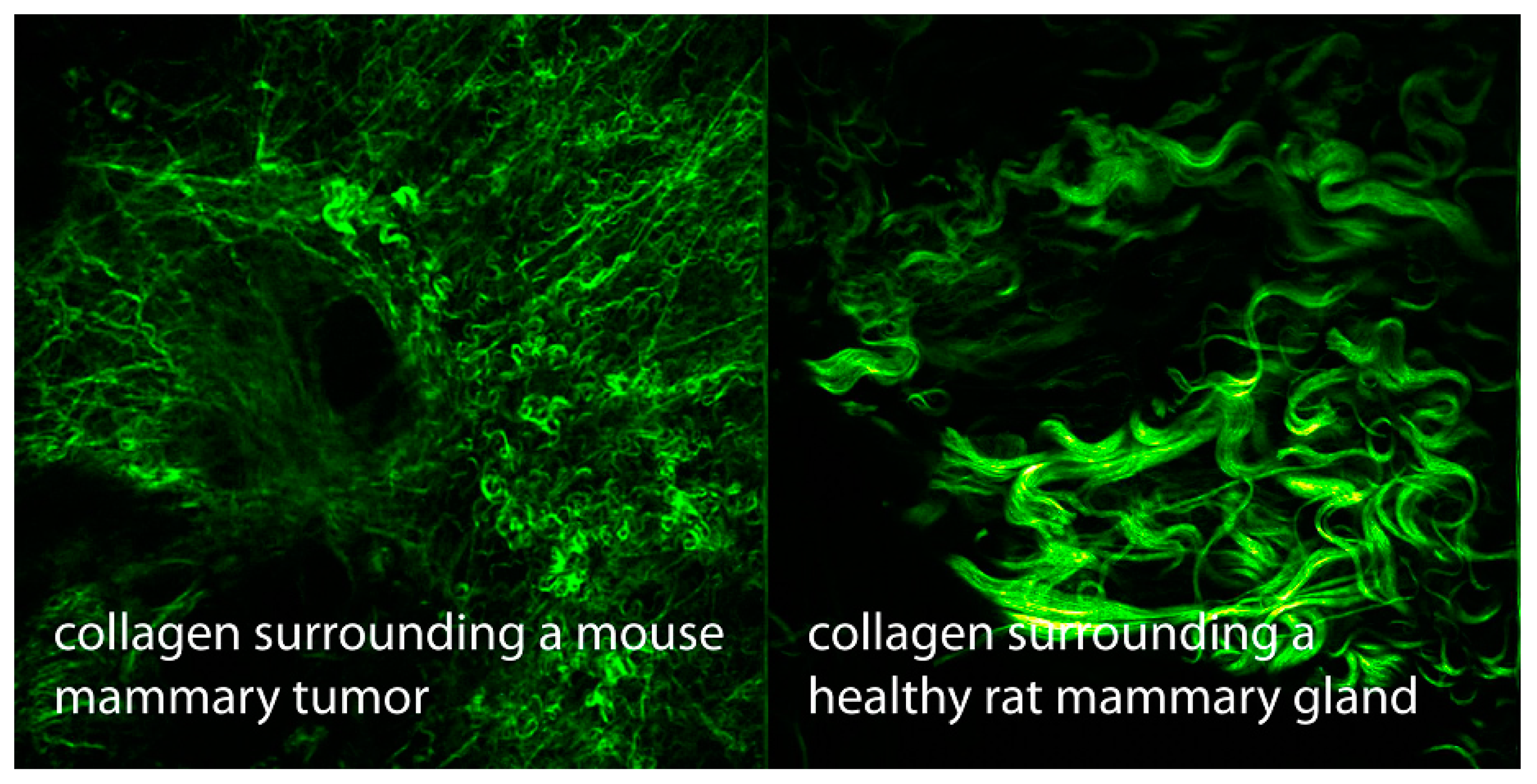
Another important property of collagen discovered in the late 1980s is its capacity for second harmonic generation (SHG),
i.e., combining photons interacting with it to form new photons with twice the energy and, therefore, double the frequency and half the wavelength [
52]. Since then,
in vivo SHG imaging has been widely developed for diagnostic purposes (
Figure 3). It should be noted that SHG was previously restricted to crystalline material, such as quartz. Although it is clear that SHG in collagen depends on hydration (with liquid crystalline water [
3]), most scientists have chosen to ignore that totally.
A paper submitted to a conference in 2003 [
54] reported the results of experiments in which Type 1 collagen bundles obtained from rat tails were structurally modified by increasing non-enzymatic cross-linking, or thermal denaturation, or by collagenase digestion, or dehydration. While they found that the hydration state significantly affected the polarization dependence of SHG, there was little or no change as a result of extensive structural modifications from cross-linking, thermal denaturation or collagenase digestion short of complete disintegration. These results strongly suggest that the liquid crystalline water adsorbed in collagen is the source of the SHG.
A second paper from a different research group published in 2005 [
55] showed that “SHG radiates from the shell of a collagen fibril rather than from its bulk.” The effective thickness of the SHG shell was strongly dependent on the ionic strength of the surrounding solution, increasing as ionic strength decreases. However, the authors have not attributed the SHG shell to liquid crystalline water.
A lot remains to be done in this fertile area (see my review [
56]). Metabolic/energetic regulation may well depend on the flow of protons via liquid crystalline water structured in nanospaces throughout the extracellular matrix (as Qi along the acupuncture meridian system) into the interior of every single cell and its nanospaces.
10. Quantum Coherent Water Orchestrating Quantum Jazz
Among Emilio’s most significant contribution for me personally is in providing a concrete hypothesis on how the staggering molecular complexities of cells and organisms can be coordinated.
In the
Rainbow Worm, I define quantum coherence after quantum physicist Roy Glauber [
57] (who later got the 2005 Nobel Prize for his work in quantum optics) in terms of factorizability. I later expressed factorizability as follows (see p.40 in [
4]): “a system is quantum coherent when its parts are so perfectly correlated that their cross-correlations factorize exactly as the product of the individual self-correlations, so that each appears paradoxically as though totally uncorrelated with the rest. It is a state of maximum local freedom
and global cohesion; something that’s impossible in a classical mechanical system.”
Quantum coherence is a sublime state of being whole: a superposition of coherent activities over all space-times, a pure (ideal) dynamic state towards which the system tends to return asymptotically. I use the idea of “quantum jazz” to highlight the immense diversity and multiplicity of supramolecular, molecular and submolecular players, the complexity and the coherence of the performance and, above all, the freedom and spontaneity, with each and every player improvising from moment to moment, yet keeping in step and in tune with the whole.
To appreciate the scope, as well as the precision and finesse of quantum jazz, we need look no further than the “natural genetic engineering” or “natural genetic modification”—cut and splice operations on DNA and RNA—that cells and organisms need to do constantly in real time in order to survive (see [
58]). Leading molecular geneticist, James Shapiro, at University of Chicago Illinois in the United States is so impressed with what he and others have been finding out over the past four decades, and especially since the human genome was sequenced, that he says evolution happens by natural genetic engineering and not by the natural selection of random mutations. In fact, there is almost nothing that’s random inside the cell and organism. Organisms are constantly adjusting to the environment by turning on and off the right genes, creating new genes if need be, shaping the environment and preparing for the future.
Just to produce a single protein, originally thought to be one continuous genetic message, requires elaborate cut and splice operations. The international research consortium project, ENCODE (Encyclopedia of DNA Elements), data have revealed that vast areas of genomic DNA include many “non-coding” segments. The “gene” is actually scattered in bits across the genome, overlapping with bits of multiple other genes that have to be spliced together to make a messenger (m)RNA for translation into a protein.
When bacteria are starved and there is a substrate they cannot metabolize in the environment, they can mutate or cut and splice to make the right genes in order to enable them to use the substrate. This phenomenon of “directed mutation” has been studied by a number of geneticists, including Shapiro. Many different proteins and DNA sequences have to come together in choreographed succession to form and rearrange the nucleoprotein complexes necessary for directing the precise cut and splice operations involved.
Geneticists are discovering more and more molecular nuts and bolts every day; the complexity of the interacting networks is enough to give anyone except the most dedicated new breed of “systems biologists” a severe headache. Genes only occupy less than 2% of the genome. The rest, thought to be useless “junk” DNA not so long ago, is 85% transcribed, and thousands of noncoding RNAs belonging to several large families with important and specific regulatory functions have been identified; and more are emerging every day. Sorting out the morass of molecules is a primary preoccupation of battalions of dedicated geneticists. The question that’s never asked is how these molecules with very specific functions can find one another and join up to do their job just at the right time and place. How does A know when and how to “recruit” B to join up with C, D, E and F to act on G at a specific site on the genome? Additionally, there are tens, if not hundreds of thousands such sites in a cell’s genome.
One answer they have not considered is electromagnetic signaling and resonance, which I have suggested since the first edition of
Rainbow Worm [
3], following Colin McClare [
59], a brilliant physiologist whom few understood, but was a major influence in my intellectual development. McClare not only pointed out the fact that resonating molecules attract one another, but also the precision with which interactions can occur, compared to the usual “lock and key” or “induced fit” hypothesis of how molecules come together due to random collisions in free diffusion. In fact, there is nothing like free diffusion possible in the living cell; it is jam packed with molecules, membranes and organelles and highly organized, largely due to self-assembly, which is probably nothing if not electromagnetic resonance at work. There is independent evidence that macromolecules sharing the same function also share a common vibrational frequency [
60].
This makes even more sense in the context of quantum electrodynamics theory. Del Giudice and colleagues [
6–
11] propose that water CDs can be easily excited and are able to capture surrounding electromagnetic fields to produce coherent excitation in the frequencies of the external fields. This, in turn, enables selective coherent energy transfer to take place. All molecules have their individual spectrum of vibrational frequencies. If the molecule’s spectrum contained a frequency matching that of the water CD, it would get attracted to the CD and become a guest participant in the CD’s coherent oscillation, settling on the surface of the CD where the CD’s excitation energy would become available to the guest molecules as activating energy for chemical reactions. Sequential reactions could occur because the new products would have a different vibrational spectrum. This could explain, in principle, how entire pathways of reactions could be assembled.
Additionally, it would also explain how directed mutations could occur [
61]. The substrate in the environment could send its electromagnetic signals to the enzyme, breaking it down, as well as to the gene encoding the enzyme, causing the gene to respond by attracting the transcription and requisite mutagenic machinery until the appropriate mutation for making an active enzyme is achieved.
Electromagnetic signals stored in CDs could also account for the “memory of water” in homeopathic remedies (see Chapter 8 in [
4]) and many other phenomena previously considered “occult”, including the importance of quantum coherent phase information underlying the clinical practice of “butterfly touch” in the last article Del Giudice wrote for
Science in Society with his wife, Margherita Tosi [
62].
Is it possible that cells or organisms as a whole also intercommunicate by means of electromagnetic and electric signals, as implied by the principle of minimal stimulus in the practice of “butterfly touch”? This is completely uncharted territory as far as conventional cell biology is concerned, but evidence for intercellular communication has existed since the 1920s and was rediscovered by many, including Fritz Popp and my friends and collaborators, Franco Musumeci, Agata Scordino and Antonio Triglia at Catania University [
63].