Infrared Cloaking, Stealth, and the Second Law of Thermodynamics
Abstract
:1. Introduction
2. Theory
2.1. Radiation Theory
2.2. Second Law of Thermodynamics
2.3. Thermodynamic Limits of Stealth and Cloaking
3. Second Law Challenges
3.1. Solid State Challenge
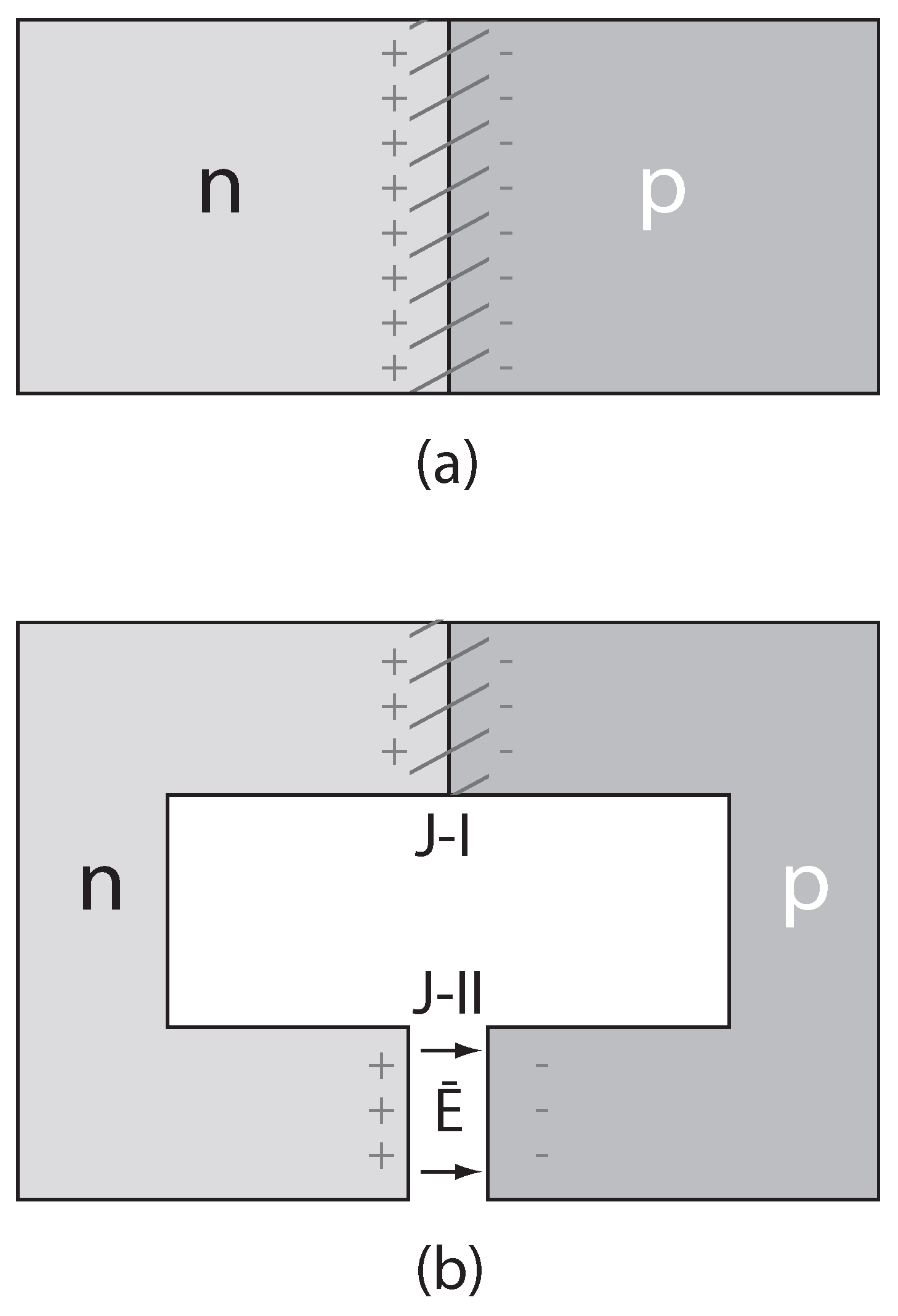
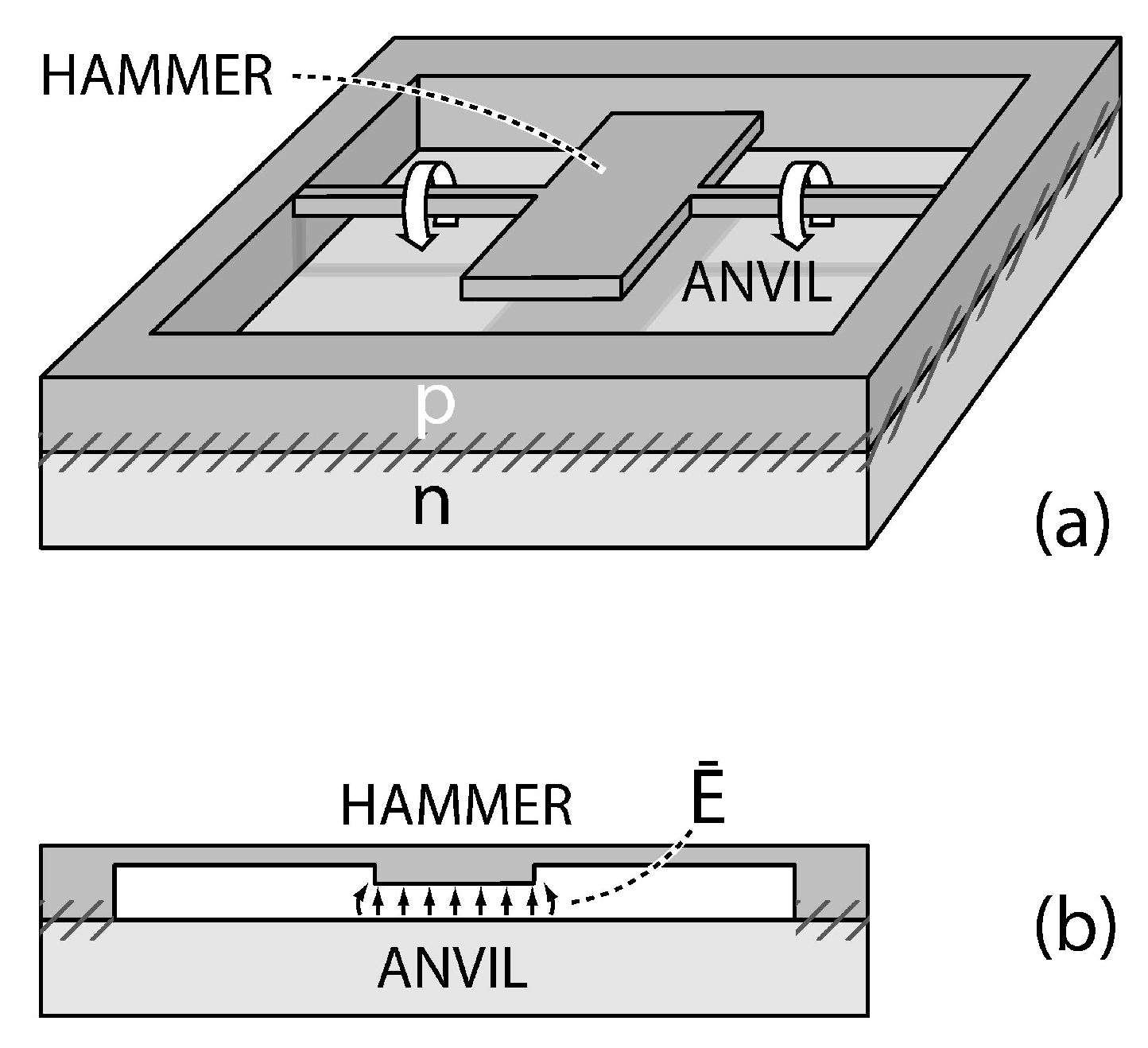
4. Strategies and Applications of SL-IRSM
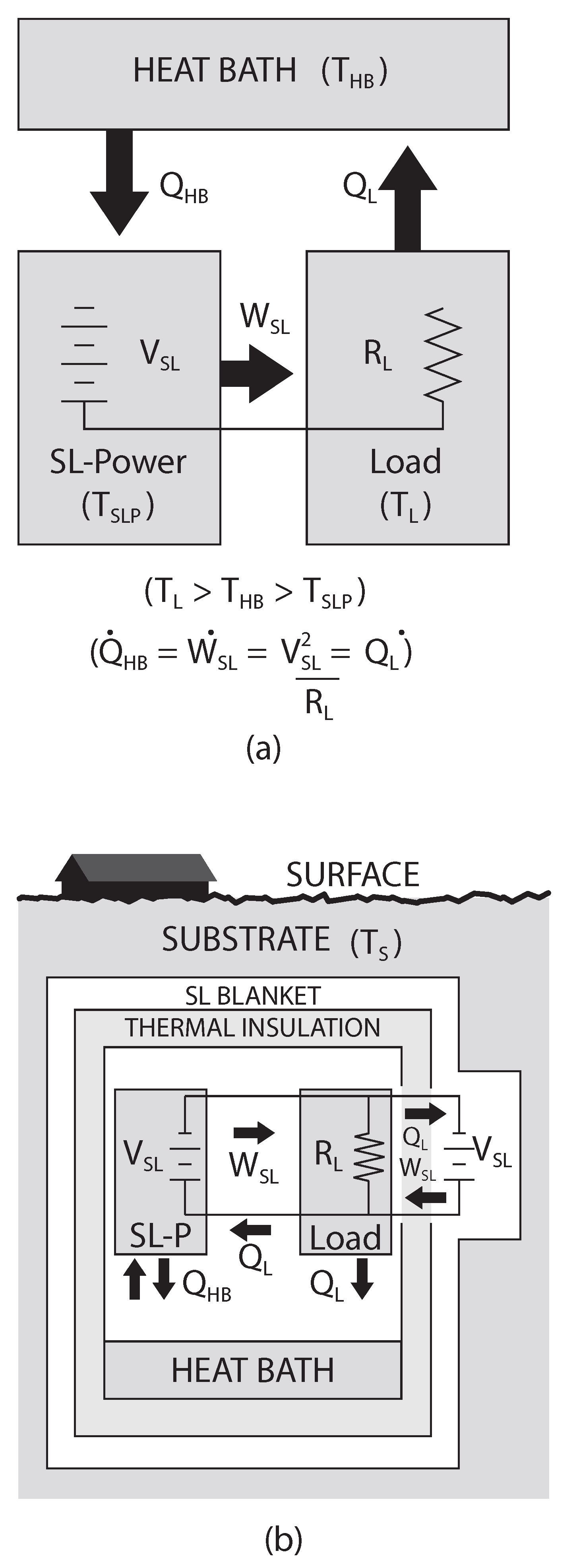
4.1. Subsurface Installation
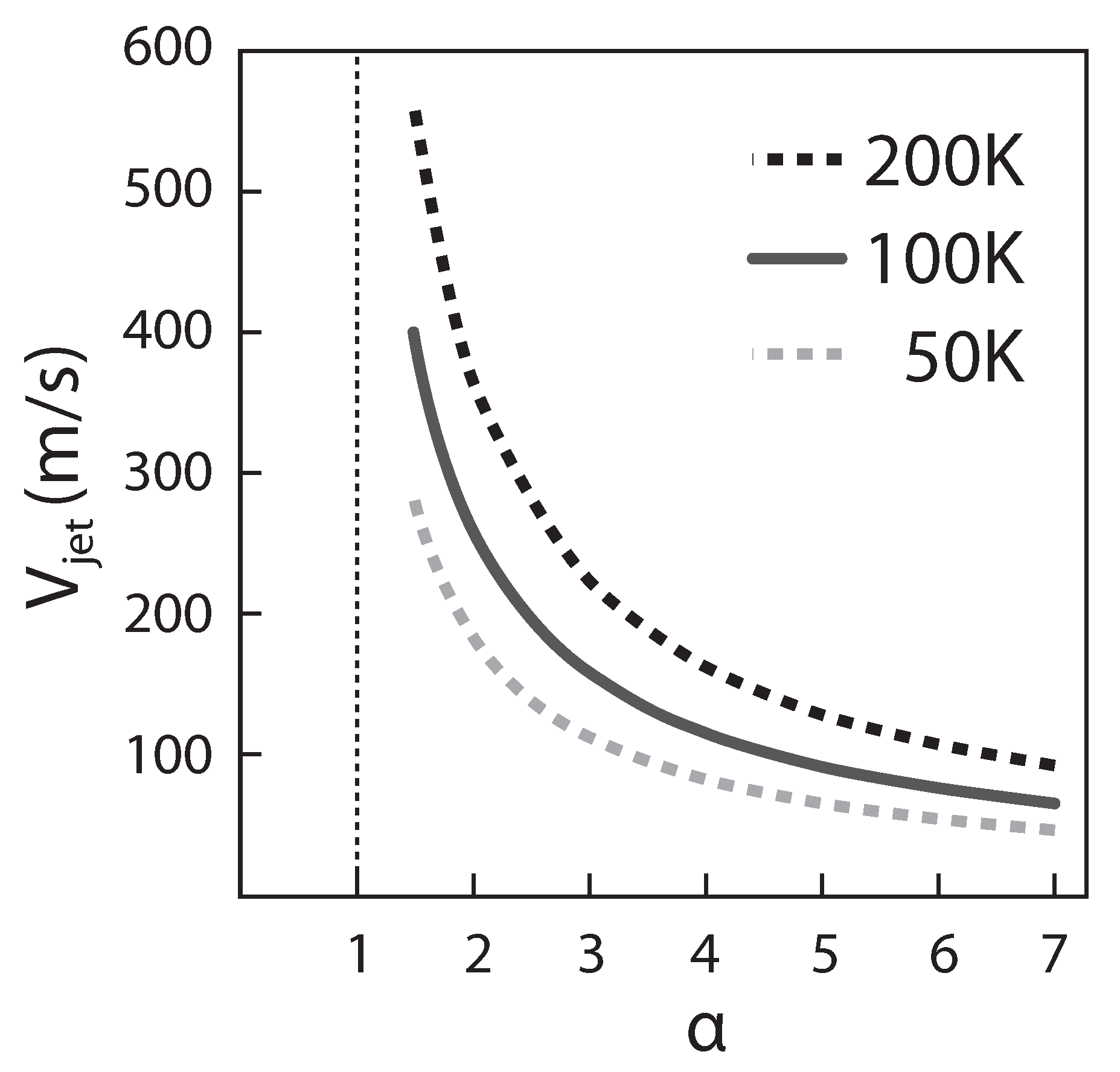
4.2. SL-Turbojet

5. Discussion, Outlook and Conclusions
Acknowledgments
References
- Jacobs, P.A. Thermal Infrared Characterization of Ground Targets and Backgrounds, 2nd ed.; SPIE Press: Bellingham, WA, USA, 2006. [Google Scholar]
- Kaplan, H. Practical Applications of Infrared Thermal Sensing and Imaging Equipment, 3rd ed.; SPIE Press: Bellingham, WA, USA, 2007. [Google Scholar]
- Mahulikar, S.P.; Sonawane, H.R.; Rao, G.A. Infrared signature studies of aerospace vehicles. Prog. Aerospace Sci. 2007, 43, 218–245. [Google Scholar] [CrossRef]
- von Baeyer, H.C. Warmth Disperses and Time Passes: The History of Heat; The Modern Library: New York, NY, USA, 1998. [Google Scholar]
- First International Conference on Quantum Limits to the Second Law; Sheehan, D.P. (Eds.) AIP Conference Proceedings. San Diego, CA, USA, 28–31 July 2002; AIP Press: Melville, NY, USA, 2002; Volume 643.
- Čápek, V.; Sheehan, D.P. Challenges to the second law of thermodynamics. In Fundamental Theories of Physics; Springer: Dordrecht, The Netherlands, 2005; Volume 146. [Google Scholar]
- Sheehan, D.P. (Ed.) The second law of thermodynamics: Foundations and status. In Proceedings of Symposium at 87th Annual Meeting of the Pacific Division of AAAS, University of San Diego, San Diego, CA, USA, 19–22 June 2006; Springer: Dordrecht, The Netherlands, 2007.
- Second Law of Thermodynamics: Status and Challenges; Sheehan, D.P. (Eds.) AIP Conference Proceedings. San Diego, CA, USA, 14–15 June 2011; AIP Press: Melville, NY, USA, 2011; Volume 1411.
- Sheehan, D.P.; Wright, J.H.; Putnam, A.R. A solid-state Maxwell demon. Found. Phys. 2002, 32, 1557–1595. [Google Scholar] [CrossRef]
- Wright, J.H.; Sheehan, D.P.; Putnam, A.R. Modeling a submicrometer electrostatic motor. J. Nanosci. Nanotech. 2003, 3, 329–334. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Wright, J.H.; Putnam, A.R.; Perttu, E.K. Intrinsically biased, resonant NEMS—MEMS oscillator and the second law of thermodynamics. Physica E 2005, 29, 87–99. [Google Scholar] [CrossRef]
- Uffink, J. Bluff your way in the second law of thermodynamics. Studies Hist. Phil. Mod. Phys. 2001, 32, 305–394. [Google Scholar] [CrossRef]
- Leff, H.S.; Rex, A.F. Maxwell’s Demon 2: Entropy, Classical and Quantum Information, Computing; Institute of Physics Publishing: Bristol, UK, 2003. [Google Scholar]
- Ergin, T.; Stenger, N.; Brenner, P.; Pendry, J.B.; Wegener, M. Three-dimensional invisibility cloak at optical wavelengths. Science 2010, 328, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Engheta, N. Plasmonic and metamaterial cloaking: Physical mechanisms and potentials. Opt. A: Pure Appl. Opt. 2008, 10, 093002. [Google Scholar] [CrossRef]
- Pendry, J.B.; Schurig, D.; Smith, D.R. Controlling electromagnetic fields. Science 2006, 312, 1780–1782. [Google Scholar] [CrossRef] [PubMed]
- Schurig, D.; Mock, J.J.; Justice, B.J.; Cummer, S.A.; Pendry, J.B.; Starr, A.F.; Smith, D.R. Metamaterial electromagnetic cloak at microwave frequencies. Science 2006, 314, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Cummer, S.A.; Popa, B.I.; Schurig, D.; Smith, D.R. Full-wave simulations of electromagnetic cloaking structures. Phys. Rev. E 2006, 74, 036621. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chettiar, U.K.; Kildishev, A.V.; Shalaev, V.M. Optical cloaking with metamaterials. Nat. Photon. 2007, 1, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, U. Optical conformal mapping. Science 2006, 312, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- Milton, G.W.; Nicorovici, N.A. On the cloaking effects associated with anomalous localized resonance. Proc. R. Soc. A 2006, 462, 3027–3059. [Google Scholar] [CrossRef]
- Milton, G.W.; Briane, M.; Willis, J.R. On cloaking for elasticity and physical equations with a transformation invariant form. New J. Phys. 2006, 8, 248. [Google Scholar] [CrossRef]
- Rumpf, R.C.; Fiddy, M.A.; Testorf, M.E. Design of generalized invisible scatterers. Opt. Express 2007, 15, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Kerker, M. Abnormally low electromagnetic scattering cross sections. J. Opt. Soc. Am. 1976, 66, 445–449. [Google Scholar]
- Hoenders, B.J. Existence of invisible nonscattering objects and nonradiating sources. J. Opt. Soc. Am. A 1997, 14, 262–266. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1983. [Google Scholar]
- Alú, A.; Engheta, N. Achieving transparency with plasmonic and metamaterial coatings. Phys. Rev. E 2005, 72, 016623. [Google Scholar] [CrossRef] [PubMed]
- Alú, A.; Engheta, N. Multifrequency optical invisibility cloak with layered plasmonic shells. Phys. Rev. Lett. 2008, 100, 113901. [Google Scholar] [CrossRef] [PubMed]
- Silveirinha, M.G.; Alú, A.; Engheta, N. Infrared and optical invisibility cloak with plasmonic implants based on scattering cancellation. Phys. Rev. B 2008, 78, 075107. [Google Scholar] [CrossRef]
- Greenleaf, A.; Kurylev, Y.; Lassas, M.; Uhlmann, G. Full-wave invisibility of active devices at all frequencies. Commun. Math. Phys. 2007, 275, 749–789. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, X.; Chan, C.T. Extending the bandwidth of electromagnetic cloaks. Phys. Rev. B 2007, 76, 241104. [Google Scholar] [CrossRef]
- Yan, M.; Ruan, Z.; Qiu, M. Cylindrical invisibility cloak with simplified material parameters is inherently visible. Phys. Rev. Lett. 2007, 99, 233901. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Yan, M.; Neff, C.W.; Qiu, M. Ideal Cylindrical cloak: Perfect but sensitive to tiny perturbations. Phys. Rev. Lett. 2007, 99, 113903. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.B. On perfect cloaking. Opt. Express 2006, 14, 12457–12466. [Google Scholar] [CrossRef] [PubMed]
- Valagiannopoulos, C.A.; Alitalo, P. Electromagnetic cloaking of cylindrical objects by multilayer or uniform dielectric claddings. Phys. Rev. B 2012, 85, 115402. [Google Scholar] [CrossRef]
- Alitalo, P.; Tretyakov, S.A. Broadband electromagnetic cloaking realized with transmission-line and waveguiding structures. Proc. IEEE 2011, 99, 1646–1659. [Google Scholar] [CrossRef]
- Valagiannopoulos, C.A.; Tsitsas, N.L. Integral equation analysis of a low-profile receiving planar microstrip antenna with a cloaking superstrate. Radio Sci. 2012, 47, RS2022. [Google Scholar] [CrossRef]
- Eddington, A. The Nature of the Physical World; Everyman’s Library & J.M. Dent: London, UK, 1942. [Google Scholar]
- Gordon, L.G.M. Brownian movement and microscopic irreversibility. Found. Phys. 1981, 11, 103–113. [Google Scholar] [CrossRef]
- Gordon, L.G.M. Maxwell’s demon and detailed balancing. Found. Phys. 1983, 13, 989–997. [Google Scholar] [CrossRef]
- Gordon, L.G.M. The molecular-kinetic theory and the second law. J. Coll. Interf. Sci 1994, 162, 512–513. [Google Scholar] [CrossRef]
- Gordon, L.G.M. The decrease in entropy via fluctuations. Entropy 2004, 6, 38–49. [Google Scholar] [CrossRef]
- Denur, J. The Doppler demon. Am. J. Phys. 1981, 49, 352–355. [Google Scholar] [CrossRef]
- Denur, J. Velocity-dependent fluctuations: Breaking the randomness of Brownian motion. Phys. Rev. A 1989, 40, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
- Denur, J. Modified Feynman ratchet with velocity-dependent fluctuations. Entropy 2004, 6, 76–86. [Google Scholar] [CrossRef]
- Denur, J. Speed-dependent weighting of the Maxwellian distribution in rarefied gases: A second-law paradox? Found. Phys. 2007, 37, 1685–1706. [Google Scholar] [CrossRef]
- Čápek, V. A thought construction of working perpetuum mobile of the second kind. Czech. J. Phys. 1996, 46, 1645–1652. [Google Scholar]
- Čápek, V. Isothermal Maxwell daemon and active binding of pairs of particles. J. Phys. A: Math. Gen. 1997, 30, 5245. [Google Scholar] [CrossRef]
- Čápek, V. Isothermal Maxwell daemon. Czech. J. Phys. 1997, 47, 845–849. [Google Scholar] [CrossRef]
- Čápek, V. Isothermal Maxwell demon as a quantum sewing machine. Phys. Rev. E 1998, 57, 3846–3852. [Google Scholar] [CrossRef]
- Čápek, V. Isothermal Maxwell daemon: Swing (fish-trap) model of particle pumping. Czech. J. Phys. 1998, 48, 879–901. [Google Scholar] [CrossRef]
- Čápek, V. From convolutionless generalized master to finite-coupling Pauli master equations. Czech. J. Phys. 1998, 48, 993–1012. [Google Scholar] [CrossRef]
- Čápek, V. Twilight of a dogma of statistical thermodynamics. Molec. Cryst. Liq. Cryst. Sci. Tech. Section A 2001, 355, 13–23. [Google Scholar] [CrossRef]
- Čápek, V.; Bok, J. Isothermal Maxwell daemon: numerical results in a simplified model. J. Phys. A: Math. Gen. 1998, 31, 8745. [Google Scholar] [CrossRef]
- Čápek, V.; Bok, J. Violation of the second law of thermodynamics in the quantum microworld. Physica A 2001, 290, 379–401. [Google Scholar] [CrossRef]
- Čápek, V.; Mančal, T. Isothermal Maxwell daemon as a molecular rectifier. Europhys. Lett. 1999, 48, 365. [Google Scholar] [CrossRef]
- Čápek, V.; Mančal, T. Phonon mode cooperating with a particle serving as a Maxwell gate and rectifier. J. Phys. A: Math. Gen. 2002, 35, 2111. [Google Scholar] [CrossRef]
- Čápek, V.; Tributsch, H. Particle (electron, proton) transfer and self-organization in active thermodynamic reservoirs. J. Phys. Chem. B 1999, 103, 3711–3719. [Google Scholar] [CrossRef]
- Čápek, V.; Frege, O. Dynamical trapping of particles as a challenge to statistical thermodynamics. Czech J. Phys. 2000, 50, 405–423. [Google Scholar] [CrossRef]
- Čápek, V.; Barvík, I. New aspects of the Davies weak coupling theory of quantum relaxation. Physica A 2001, 294, 388–402. [Google Scholar] [CrossRef]
- Čápek, V.; Sheehan, D.P. Quantum mechanical model of a plasma system: A challenge to the second law of thermodynamics. Physica A 2002, 304, 461–479. [Google Scholar] [CrossRef]
- Allahverdyan, A.E.; Nieuwenhuizen, Th.M. Extraction of work from a single thermal bath in the quantum regime. Phys. Rev. Lett. 2000, 85, 1799. [Google Scholar] [CrossRef] [PubMed]
- Allahverdyan, A.E.; Nieuwenhuizen, Th.M. Breakdown of the Landauer bound for information erasure in the quantum regime. Phys. Rev. E 2001, 64, 056117. [Google Scholar] [CrossRef] [PubMed]
- Allahverdyan, A.E.; Nieuwenhuizen, Th.M. Testing the violation of the Clausius inequality in nanoscale electric circuits. Phys. Rev. B 2002, 66, 115309. [Google Scholar] [CrossRef]
- Allahverdyan, A.E.; Nieuwenhuizen, Th.M. Statistical thermodynamics of quantum Brownian motion: Construction of perpetuum mobile of the second kind. Phys. Rev. E 2002, 66, 036102. [Google Scholar]
- Allahverdyan, A.E.; Nieuwenhuizen, Th.M. Bath-generated work extraction and inversion-free gain in two-level systems. J. Phys. A: Math. Gen. 2003, 36, 875. [Google Scholar] [CrossRef]
- Berger, J. The Chernogolovka experiment. Physica E 2005, 29, 100–103. [Google Scholar] [CrossRef]
- Berger, A nonconventional scenario for thermal equilibrium. Found. Physics 2007, 37, 1738–1743. [CrossRef]
- Crosignani, B.; Di Porto, P. Approach to thermal equilibrium in a system with adiabatic constraints. Am. J. Phys. 1996, 64, 610. [Google Scholar] [CrossRef]
- Crosignani, B.; Di Porto, P. On the validity of the second law of thermodynamics in the mesoscopic realm. Europhys. Lett. 2001, 53, 290. [Google Scholar] [CrossRef]
- Crosignani, B.; Di Porto, P. Random fluctuations of diathermal and adiabatic pistons. Found. Physics 2007, 37, 1707–1715. [Google Scholar] [CrossRef]
- Crosignani, B.; Di Porto, P.; Conti, C. The adiabatic piston and the second law of thermodynamics. In Quantum Limits to the Second Law; AIP Press: Melville, NY, USA, 2002; Volume 643, p. 267. [Google Scholar]
- Crosignani, B.; Di Porto, P.; Conti, C. The adiabatic piston: A perpetuum mobile in the mesoscopic realm. Entropy 2004, 6, 50–56. [Google Scholar] [CrossRef]
- Keefe, P. Coherent magneto-caloric effect heat engine process cycle. In Quantum Limits to the Second Law; AIP Press: Melville, NY, USA, 2002; Volume 643, p. 213. [Google Scholar]
- Keefe, P. Coherent magneto-caloric effect superconductive heat engine process cycle. J. Mod. Optics 2003, 50, 2443–2454. [Google Scholar] [CrossRef]
- Keefe, P. Second law violation by magneto-caloric effect adiabatic phase transition of type I superconductive particles. Entropy 2004, 6, 116–127. [Google Scholar] [CrossRef]
- Nikulov, A.V.; Zhilyaev, I.N. The Little-Parks effect in an inhomogeneous superconducting ring. J. Low Temp. Phys. 1998, 112, 227–235. [Google Scholar] [CrossRef]
- Nikulov, A.V. Quantum force in a superconductor. Phys. Rev. B 2001, 64, 012505. [Google Scholar] [CrossRef]
- Dubonos, S.V.; Kuznetsov, V.I.; Zhilyaev, I.N.; Nikulov, A.V. Observation of the external-ac-current-induced dc voltage proportional to the steady current in superconducting loops. JETP Lett. 2003, 77, 371–375. [Google Scholar] [CrossRef]
- Miller, S.L. Insights into the second saw of thermodynamics from anisotropic gas-surface interactions. Found. Phys. 2007, 37, 1660–1684. [Google Scholar] [CrossRef]
- Sheehan, D.P. A paradox involving the second law of thermodynamics. Phys. Plasmas 1995, 2, 1893. [Google Scholar] [CrossRef]
- Sheehan, D.P. Response to comment on “A paradox involving the second law of thermodynamics”. Phys. Plasmas 1996, 3, 706. [Google Scholar] [CrossRef]
- Sheehan, D.P. Another paradox involving the second law of thermodynamics. Phys. Plasmas 1996, 3, 104. [Google Scholar] [CrossRef]
- Sheehan, D.P. Four paradoxes involving the second law of thermodynamics. J. Sci. Explor. 1998, 12, 303. [Google Scholar]
- Sheehan, D.P. Dynamically-maintained, steady-state pressure gradients. Phys. Rev. E 1998, 57, 6660. [Google Scholar] [CrossRef]
- Sheehan, D.P. Reply to “Comment on ‘Dynamically maintained steady-state pressure gradients”. Phys. Rev. E 2000, 61, 4662. [Google Scholar] [CrossRef]
- Sheehan, D.P. The second law and chemically-induced, steady-state pressure gradients: Controversy, corroboration and caveats. Physica A 2001, 280, 185. [Google Scholar] [CrossRef]
- Sheehan, D.P. Thermosynthetic life. Found. Phys. 2007, 37, 1774. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Means, J.D. Minimum requirement for second law violation: A paradox revisited. Phys. Plasmas 1998, 5, 2469. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Glick, J.; Means, J.D. Steady-state work by an asymmetrically inelastic gravitator in a gas: A second law paradox. Found. Phys. 2000, 30, 1227. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Glick, J. Gravitationally-induced, dynamically-maintained, steady-state pressure gradients. Phys. Script. 2000, 61, 635. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Glick, J.; Duncan, T.; Langton, J.A.; Gagliardi, M.J.; Tobe, R. Phase space portraits of an unresolved Maxwell demon. Found. Phys. 2002, 32, 441. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Glick, J.; Duncan, T.; Langton, J.A.; Gagliardi, M.J.; Tobe, R. Phase space analysis of a gravitationally-induced, steady-state nonequilibrium. Phys. Scripta 2002, 65, 430. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Seideman, T. Intrinsically biased electrocapacitive catalysis. J. Chem. Physics 2005, 122, 204713. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.P.; Gross, D.H.E. Extensivity and the thermodynamic limit: Why size really does matter. Physica A 2006, 370, 461. [Google Scholar] [CrossRef]
- Sheehan, D.P. Energy, entropy and the environment (How to increase the first by decreasing the second to save the third). J. Sci. Explor. 2008, 22, 459. [Google Scholar]
- Neudeck, G.W. Volume II: The pn Junction Diode , 2nd ed.; In Modular Series on Solid State DevicesPierret, R.F., Neudeck, G.W., Eds.; Addison-Wesley: Reading, UK, 1989. [Google Scholar]
- Sadewasser, S.; Glatzel, Th.; Shikler, R.; and Rosenwaks, Y. Resolution of Kelvin probe force microscopy in ultrahigh vacuum: comparison of experiment and simulation. Appl. Surf. Sci. 2003, 210, 32–36. [Google Scholar] [CrossRef]
- Kikukawa, A.; Hosaka, S.; Imura, R. Vacuum compatible high-sensitive Kelvin probe force microscopy. Rev. Sci. Instrum. 1995, 67, 1463–1467. [Google Scholar] [CrossRef]
- Sheehan, D.P.; Wright, J.H. Experimental measurements of electric fields in diodic air gaps: Toward a second law challenge. In Second Law of Thermodynamics: Status and Challenges. AIP Conference Proceedings, San Diego, CA, USA, 14–15 June 2011; AIP Press: Melville, NY, USA, 2011; Volume 1411, pp. 147–157. [Google Scholar]
- Bienstman, J.; Vandewalle, J.; Puers, R. The autonomous impact resonator: A new operating principle for a silicon resonant strain gauge. Sensor. Actuator. Phys. 1998, 66, 40–49. [Google Scholar] [CrossRef]
- Roukes, M.L. Nanoelectromechanical systems. In Proceedings of the Technical Digest of the 2000 Solid-State Sensor and Actuator Workshop, Hilton Head Island, SC, USA, 4–8 June 2000.
- Pelesko, J.A.; Bernstein, D.H. Modeling MEMS and NEMS; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Nanotechnology; Timp, G. (Ed.) Springer-Verlag: New York, NY, USA, 1999.
- Sheehan, D.P.; Wright, J.H. Heat recyclers for interstellar travel. In Presented at the 100 Year Starship Conference, Orlando, Fl, USA, 30 September–2 October 2011.
© 2012 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sheehan, D.P. Infrared Cloaking, Stealth, and the Second Law of Thermodynamics. Entropy 2012, 14, 1915-1938. https://doi.org/10.3390/e14101915
Sheehan DP. Infrared Cloaking, Stealth, and the Second Law of Thermodynamics. Entropy. 2012; 14(10):1915-1938. https://doi.org/10.3390/e14101915
Chicago/Turabian StyleSheehan, Daniel P. 2012. "Infrared Cloaking, Stealth, and the Second Law of Thermodynamics" Entropy 14, no. 10: 1915-1938. https://doi.org/10.3390/e14101915



